Biopharmaceutical Glycosylation Site Analysis Service
- N-glycosylation Analysis
- O-glycosylation Analysis
- Glycan Structure and Composition Analysis
- Monosaccharide Composition Analysis
- High Sensitivity: Detects even low-abundance glycosylation sites.
- High Accuracy: Ensures precise localization of glycosylation sites and a thorough analysis of glycan structures.
- Customized Services: Tailored analysis plans to meet specific customer requirements.
- Comply with ICH Q6B guidelines to ensure biopharmaceutical samples meet new market standards.
- Analyze glycosylation profiles across a variety of proteins, antibodies, and cells.
- Identify and study glycan biomarkers.
- Compare glycosylation patterns across different samples.
- Detect and characterize unusual glycosylation events.
- Maintain batch-to-batch consistency during production.
Glycosylation significantly influences the three-dimensional structure, biological activity, transport, and localization of proteins. In biopharmaceutical manufacturing, particularly in the production of protein-based drugs, glycosylation is crucial for maintaining the intended structural integrity and functional performance of pharmaceuticals. Even slight variations in glycan chains can substantially affect a drug's effectiveness and safety. As biopharmaceutical innovation progresses, thorough and precise glycosylation analysis becomes critical for both manufacturers and regulatory bodies. Mass spectrometry has emerged as an effective analytical tool for glycosylation, encompassing stages from drug development to the final batch release.
MtoZ Biolabs employs state-of-the-art mass spectrometry alongside expert bioinformatics to offer glycosylation site analysis for biopharmaceuticals. These analyses not only verify glycosylation sites within products but also assess the glycan chains' diversity and complexity, providing accurate scientific support for drug design and quality control. Additionally, our services elucidate glycosylation's specific impact on drug functions, enhancing drug efficacy and safety while meeting stringent regulatory standards.
Analysis Workflow
1. Sample Preparation
Proteins are degraded to release glycopeptides.
2. Enrichment and Separation
Specialized enrichment columns are utilized for the effective separation of glycopeptides.
3. Mass Spectrometry Analysis
High-resolution mass spectrometry is employed for comprehensive analysis.
4. Data Processing
Advanced bioinformatics tools are used for data interpretation, ensuring results are both accurate and reliable.
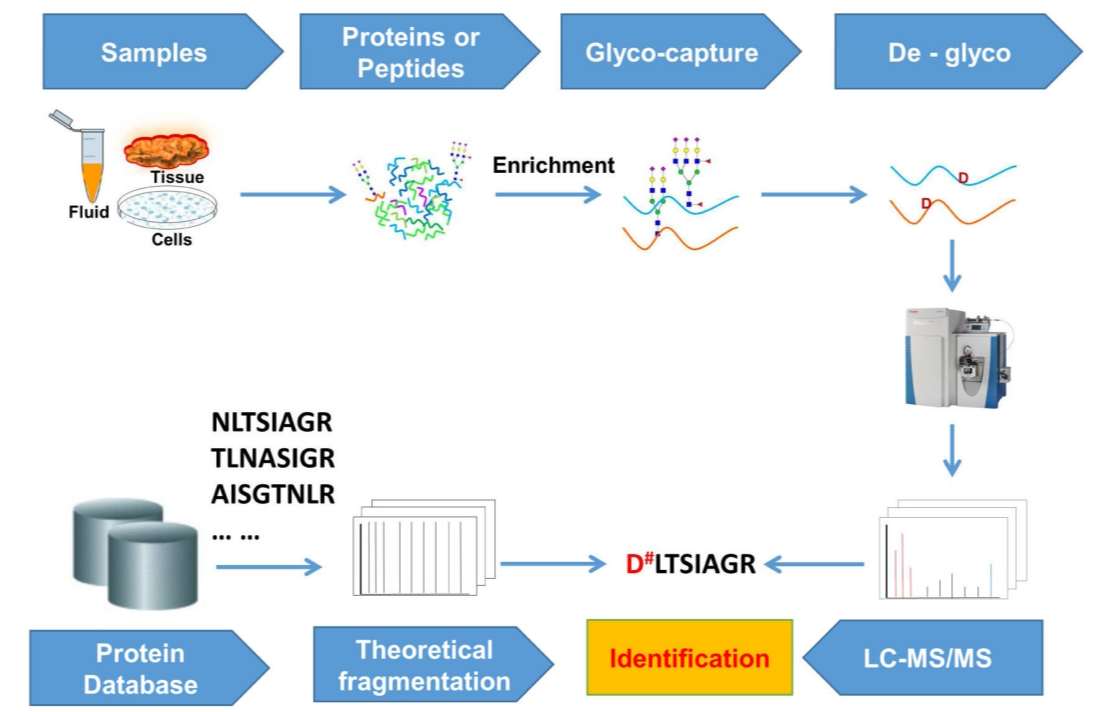
Dang, L. Y. et al. Trends Anal. Chem. 2019.
Figure 1. Analysis Workflow of N-linked Glycoprotein Site Identification
Services at MtoZ Biolabs
Service Advantages
Applications
Deliverables
1. Experimental Procedures
2. Relevant Mass Spectrometry Parameters
3. Detailed Information on Glycosylation Site
4. Mass Spectrometry Images
5. Raw Data
How to order?







