Biopharmaceutical Stability Analysis Service
Biopharmaceutical stability analysis is crucial for assessing the safety, efficacy, and quality of biopharmaceuticals throughout their storage, transportation, and usage. This analysis evaluates changes in the chemical, physical, microbiological, and biological properties of the drugs.
Biopharmaceutical stability can be categorized into various types based on the testing objectives and conditions, including:
1. Accelerated Stability
Conducted at temperatures above normal storage conditions to predict the drug's shelf-life and stability under standard storage conditions.
2. Long-Term Stability
Carried out at recommended storage temperatures, typically at 25°C, to determine the drug's shelf life.
3. Intermediate Stability
Performed at temperatures between accelerated and long-term conditions to yield quicker results.
4. Real-Time Stability
Conducted under actual storage conditions to monitor the drug's stability during use.
5. Photostability Testing
Assesses the effects of light, including direct sunlight, near UV, and visible light, on drug stability.
6. Humidity Stability Testing
Evaluates drug stability under varying humidity levels.
7. Freezing-Thawing Stability Testing
Assesses drug stability during freeze-thaw cycles.
8. Transport Stability Testing
Simulates potential transport conditions to assess drug stability.
9. Use Stability Testing
Evaluates stability after the drug has been opened or used multiple times.
10. Kinetic Stability Testing
Employs kinetic models to analyze temporal changes in drug degradation to determine degradation rates and stability indicators.
11. Compatibility Stability Testing
Assesses stability after exposure to other drugs, excipients, or packaging materials.
12. Microbial Stability Testing
Ensures that sterile or non-sterile products keep their microbial content under the limited content throughout their lifespan.
13. Physical Stability Testing
Evaluates changes in the drug's physical characteristics, such as crystallinity and particle size distribution, during storage.
14. Chemical Stability Testing
Detects chemical changes during storage, including the formation of degradation products.
15. Thermal Cycling Testing
Simulates daily temperature variations to assess stability under temperature changes.
16. Stress Testing
Conducted under extreme conditions to evaluate the drug's inherent stability and potential degradation pathways.
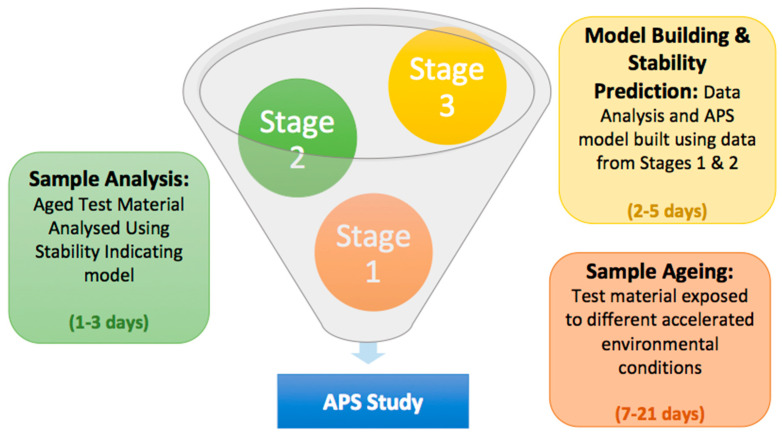
Figure 1. Schematic Diagram of the Accelerated Stability Research Stage [1]
Testing Content Required by Policies and Regulations
CVM GFI #5 Drug Stability Guidelines Drug Stability Guidelines
1. General Considerations
(1) Protocols: Applicants should submit stability testing protocols for review before submitting new or supplemental NADA.
(2) General Stability Considerations: This section addresses the definition of active ingredients, potency, drug preparation, chemical and physical properties, additives, product changes, relevance to efficacy and toxicity studies, degradation products, and product stability parameters.
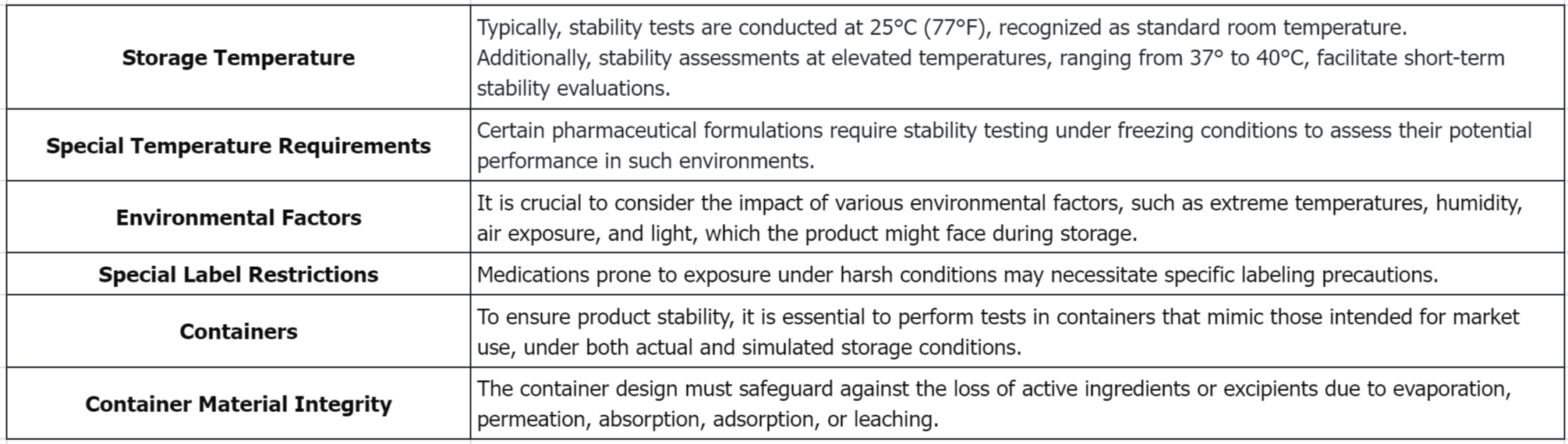
(3) Storage Conditions: This includes specifications for shelf-life duration of studies, expiration dates, temperature settings, environmental factors, and special labeling restrictions.
(4) Containers: The discussion on container covers container material integrity, containers for liquids: special considerations, physical observations, sealed containers, adhesive/glue, and container changes.
(5) Stability Samples: Details on samples, sampling plan, handling and analytical processes are provided.
(6) Stability Schedule: Provides general information on test schedules and recommendations for optimal testing timelines.
(7) Analytical Methods: Discusses quality control and release methods, methods attributes, method controls and conditions, standard curves, and stability method references.
(8) Statistical Evaluation: Focuses on design considerations, data analysis, and expiration date determination.
(9) Reporting of Data: Outlines stability study commitments and stability study commitment format.
2. Specific Considerations
Covers the stability testing for pharmaceutical dosage forms including solids, sterile formulations, Types A, B, and C drug products, drug composition, soluble powders and drinking water, oral drenches, milk replacers, mastitis preparations, sustained-release products, microencapsulated products, vat dips, rDNA products, and drug substances.
3. Specific Indicators to be Tested
4. Stability Testing Conditions
Biological Drug Stability Analysis Solutions
Multiple stability tests can be provided, including influencing factor tests, accelerated tests, and long-term tests.
Detect the following indicators:
Service Advantages
1. Provides Specialized and Reliable Services that Encompass a Broad Spectrum of Drug Stability Assessments
2. Offers High Cost-Effectiveness in Testing with Rapid Turnaround Times and Detailed, Trustworthy Reports
3. Features an Array of Advanced Equipment, Including Multiple High-Resolution Mass Spectrometers
Case Study
1. Formulation and Stability Testing of Photolabile Drugs
Exposure of drugs to irradiation impacts the stability of their formulations, altering their physicochemical properties. The effects of common stabilizers and excipients can be difficult to predict, underscoring the importance of stability testing in final formulations. The selection of protective packaging should be guided by an understanding of the wavelengths that induce instability. Additionally, acquiring comprehensive details about a drug's photo-reactivity can facilitate the development of photo-responsive drug delivery systems to minimize adverse effects and/or optimize drug targeting. A paper delved into the practical issues concerning the formulation and stability testing of photolabile drugs.
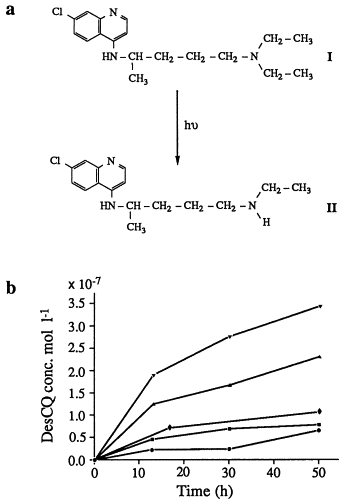
Figure 2. Correlation Between Irradiation Time and Degradation Products in Polymorphic Chloroquine [2]
2. Stability of Penicillin in Prefilled Syringes for Drug Allergy Skin Tests
A study assessed the stability of penicillin, commonly used in skin prick tests (SPT) and intradermal tests (IDT), within prefilled syringes as required by drug allergy protocols. Findings indicated that these syringes can be aseptically prepared well before use and remain stable when stored at 4°C for up to 2 to 7 days, facilitating a change in clinical setting. This enabled pharmacies to pre-prepare reagents for penicillin allergy testing, enhancing safety and efficiency.
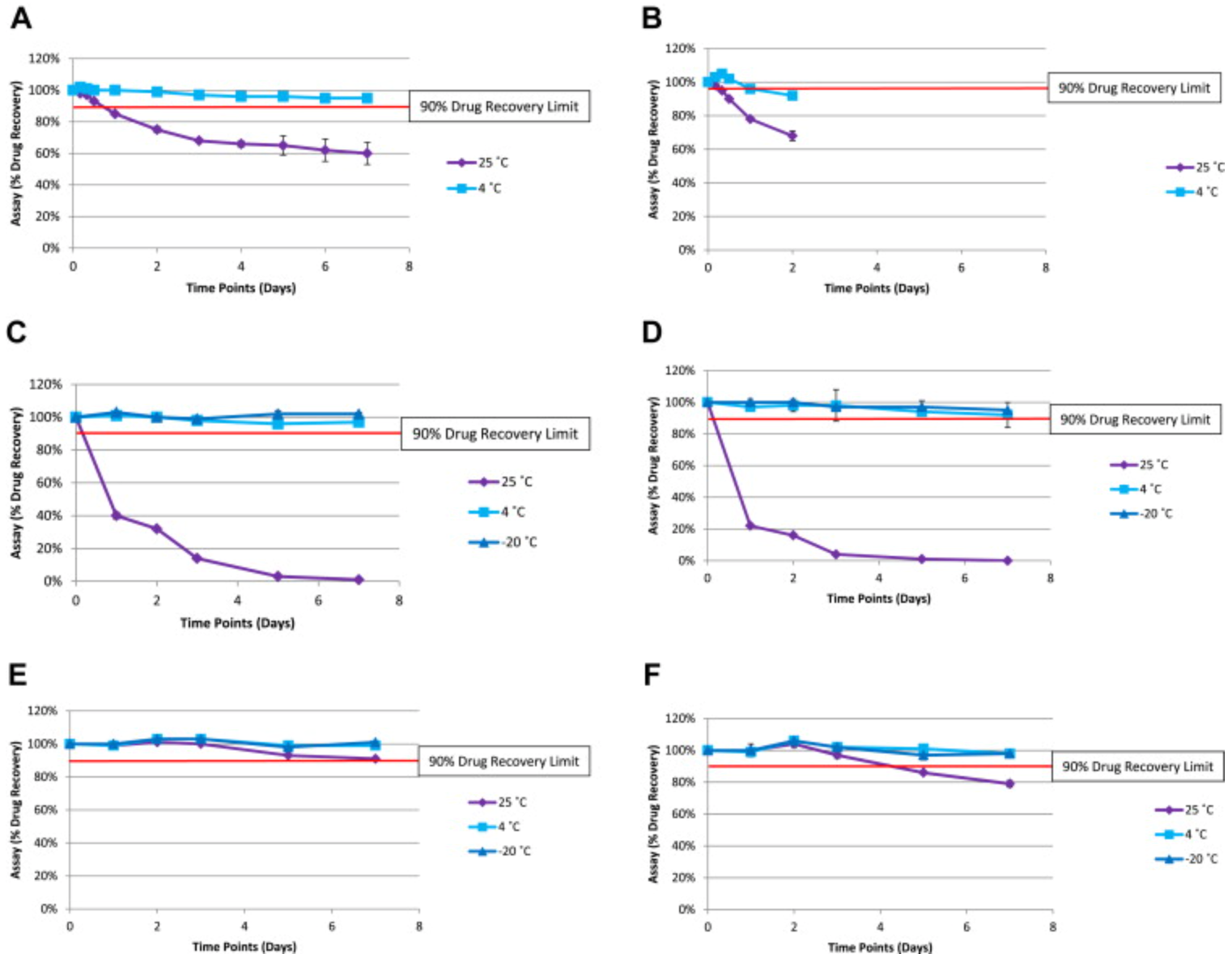
Figure 3. Stability Data for Amoxicillin Sodium (2 mg/mL and 20 mg/mL), Benzylpenicillin Sodium (0.6 mg/mL and 6 mg/mL), and Flucloxacillin Sodium (0.5 mg/mL and 5 mg/mL) [3]
3. Experimental Design and Multivariate Analysis Methods to Study the Dissolution Stability of Modified-Release Drug Products to Support Lean Design Strategies
A paper introduced experimental design and multivariate analysis for evaluating the stability of modified-release drug products, aimed at enhancing post-approval lean stability approaches. It focused on investigating the underlying causes for accelerated dissolution when stability conditions were applied. Stability data were statistically analyzed using multiple linear regression statistics. The MODDE 12.1 software was utilized to model the design space for these studies. Key experimental parameters included temperature, time, packaging, batch number, and suppliers of active pharmaceutical ingredients. Through multple linear regression modeling of the stability data collected over six months, researchers identified and confirmed the stability-related quality attributes and shelf-life limiting attributes. The analysis of multiple linear regression correlation coefficients helped pinpoint how the decrease in antioxidant during stability caused potential shift in dissolution. Nonetheless, the major contributors to accelerated dissolution were identified as other material and process variables. Researchers demonstrated how these methodologies were beneficial in fostering lean stability approaches. Leveraging their enhanced understanding of pharmaceuticals, researchers conducted two scaled-down long-term stability studies. These demonstrated that even with a 50% reduction in design, a 24-month shelf life could be confidently asserted. Adopting quality-by-design approaches in stability studies can minimize unnecessary analytical testing, facilitate meaningful data interpretation, and enable the formulation of risk-based stability testing strategies.
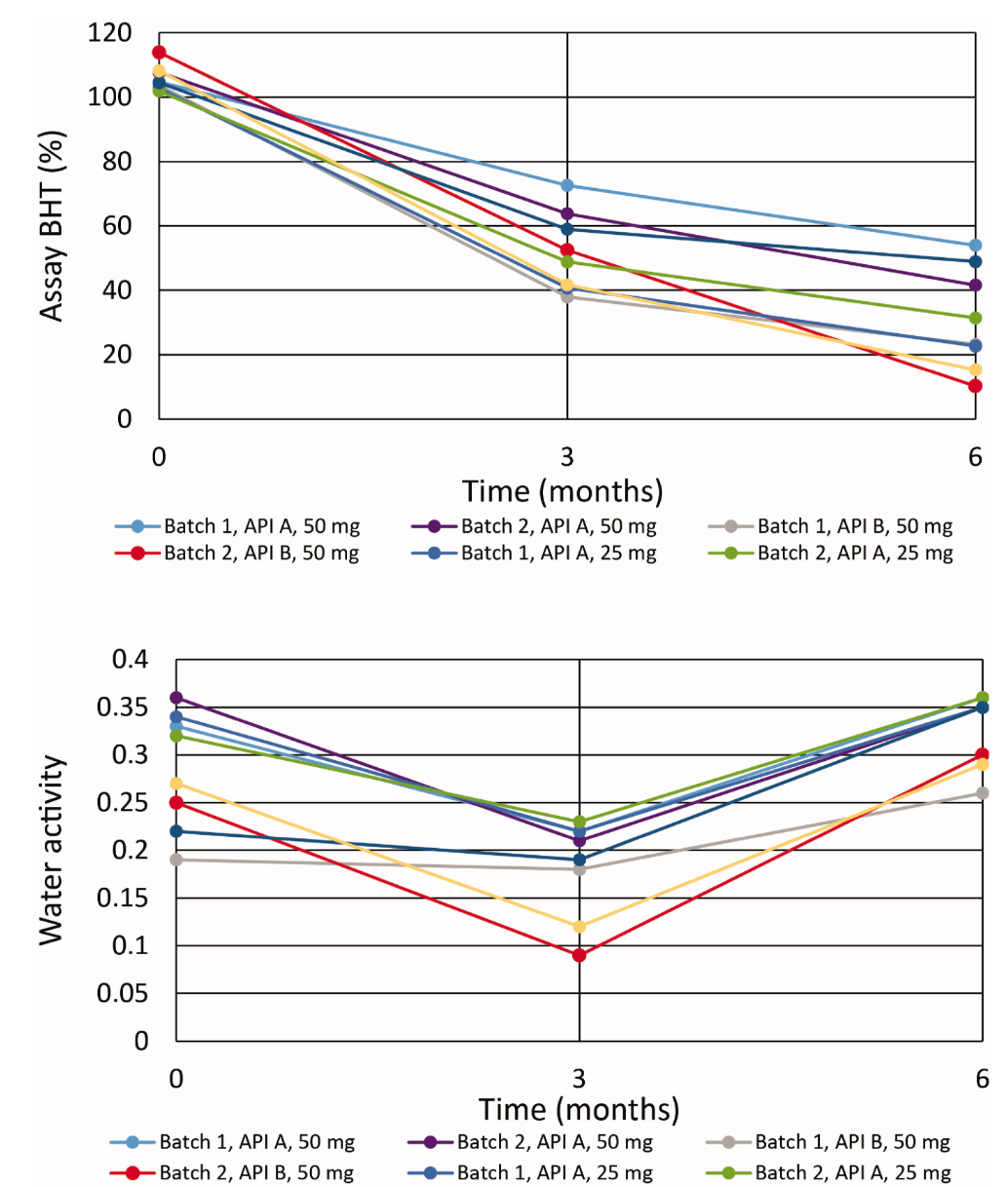
Figure 4. Accelerated Stability Study Results for Drug BHT in HDPE.30 Bottles (Top Image) and Water Activity (Bottom Image) [4]
References
[1] González-González O, Ramirez IO, Ramirez BI, O'Connell P, Ballesteros MP, Torrado JJ, Serrano DR. Drug Stability: ICH versus Accelerated Predictive Stability Studies. Pharmaceutics. 2022 Oct 28;14(11):2324. doi: 10.3390/pharmaceutics14112324. PMID: 36365143; PMCID: PMC9693625.
[2] Tønnesen HH. Formulation and stability testing of photolabile drugs. Int J Pharm. 2001 Aug 28;225(1-2):1-14. doi: 10.1016/s0378-5173(01)00746-3. PMID: 11489550.
[3] Garg A, Chan D, Ambados F, Lwin E, Song Y, Garg S. Penicillin stability in prefilled syringes for the purpose of skin testing for drug allergy. J Allergy Clin Immunol Pract. 2015 Jul-Aug;3(4):599-601. doi: 10.1016/j.jaip.2015.01.015. Epub 2015 Mar 7. PMID: 25758915.
[4] Jordan N, Roškar R, Grabnar I. Design of experiments and multivariate analysis approach to study dissolution stability of a modified-release drug product to support lean design strategies. Drug Dev Ind Pharm. 2021 Sep;47(9):1481-1488. doi: 10.1080/03639045.2021.2001491. Epub 2021 Nov 17. PMID: 34726551.
How to order?







