Antibody Drug Analysis Service
Antibody therapies leverage human immune system antibodies to treat diseases by targeting specific proteins on pathogens or abnormal cells with exceptional precision. The development of antibody therapies has evolved from early polyclonal sera to monoclonal antibodies (mAbs), and now to genetically engineered antibodies, focusing on enhancing drug safety, effectiveness, and manufacturing efficiency.
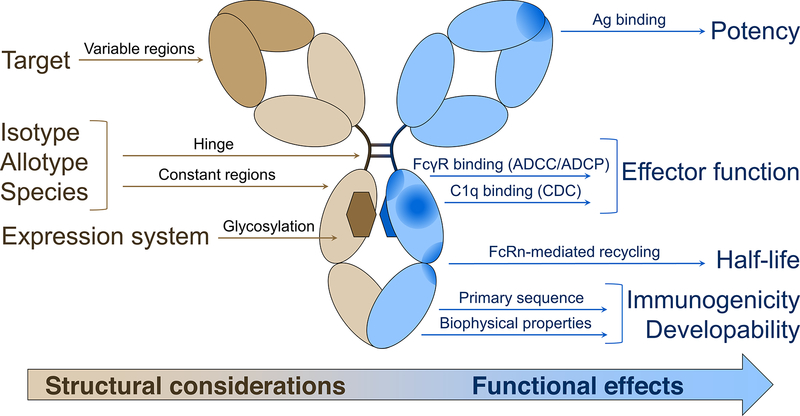
Figure 1. Structural Considerations Based on IgG Therapy Drug Design and Their Impact on Biological and Clinical Functions [1]
Antibody therapies are categorized based on structural features, mechanisms of action, and clinical uses. Key types include:
1. Monoclonal Antibodies (mAbs)
Predominantly used antibody type, derived from a single B-cell clone, characterized by high specificity and uniformity. They are crucial in treating diseases like cancer and autoimmune disorders by targeting specific antigens.
2. Antibody Fusion Proteins
Antibody drugs of this type enhance therapeutic outcomes by combining antibodies with other proteins or peptides that have therapeutic properties. For instance, certain fusion proteins exhibit both the targeted specificity typical of antibodies and the catalytic activity characteristic of enzymes.
3. Antibody-Drug Conjugates (ADCs)
Antibody-drug conjugates (ADCs) consist of antibodies linked to cytotoxic drugs via a linker, allowing for the precise delivery of cytotoxic agents to targeted cells or tissues, leveraging the specificity of the antibody.
4. Bispecific Antibodies (BsAbs)
Capable of recognizing two distinct antigens simultaneously, these antibodies are used for dual targeting strategies in cancer therapy, potentially activating the immune system against tumors.
5. Multi-Specific Antibodies
Besides BsAbs, there are also Antibodies that can target multiple antigens simultaneously, such as Tri-specific Antibodies and Tetra-specific Antibodies.
6. Antibody Fragments
These components are smaller segments of antibodies, such as single-chain antibodies (scFv) and Fab fragments, which are known for their enhanced tissue penetration and accelerated clearance from the body.
7. Radionuclide Antibody Conjugates (RACs)
These therapies combine therapeutic antibodies with radioactive isotopes to treat diseases, primarily certain cancers, utilizing the isotopes' radioactive effects.
Other types of antibody drugs: with the development of technology, many new antibody drugs are being developed, such as antibody mimics, antibody drug conjugates (such as antibody-small molecule drug conjugates), etc.
Advantages and Limitations of Antibody Drugs as Therapeutic Drugs
1. Advantages
(1) High Target Specificity: Antibody drugs can specifically recognize and bind to disease-related targets, such as particular proteins on the surfaces of pathogens or abnormal cells. Their exceptional specificity enables them to precisely target diseased cells or pathological processes, thereby minimizing the effects on healthy cells and reducing side effects.
(2) Significant Efficacy: Antibody drugs, due to their precise targeting capabilities, are exceptionally effective at killing cancer cells and inhibiting pathological processes, thereby enhancing treatment efficacy. For instance, the success of Enhertu (trastuzumab-drug conjugates) highlights the substantial potential of ADCs in cancer therapy, establishing a new standard in this area.
(3) Reduced Side Effects: Antibody drugs are less toxic to normal cells compared to traditional chemotherapy, leading to fewer side effects due to their targeted action.
(4) Improved Treatment Experience: The reduced side effects from antibody treatments contribute to a more positive overall therapy experience for patients.
2. Limitations
(1) Poor Pharmacokinetic Properties: Many antibody drugs have a short half-life in the body, requiring frequent dosing. This may increase adverse side effects and could lead to high costs and compliance issues for patients and the healthcare system.
(2) Challenges in Manufacturing and Storage: The production of antibody drugs involves many steps, including screening for high-expression cell lines, development of culture media, and development of purification technologies. How to shorten the R&D cycle and reduce production costs are important factors limiting the development of antibody drug research.
(3) Immunogenicity Risks: Antibody drugs, particularly those derived from non-human sources like mouse antibodies, can trigger immune responses in humans, which may lead to reduced effectiveness and potential safety concerns.
(4) Limited Tissue Accessibility: The effectiveness of antibody drugs is sometimes limited by their large size, which restricts their ability to penetrate and accumulate in certain tissues, potentially limiting their therapeutic application.
(5) Stability Concerns: Throughout their manufacturing, transportation, and storage phases, antibody drugs may encounter conditions that lead to instability, such as aggregation or denaturation, compromising their effectiveness and safety.
Strategies to Overcome Limitations Associated with Antibody Drugs
1. Enhancing Drug Targeting and Affinity
Through affinity maturation techniques, the binding strength between antibodies and antigens is enhanced. This includes random mutation, directed mutation, semi-rational mutation, and antibody fusion technologies to obtain antibodies with higher specificity and affinity.
2. Enhancing Antibody Stability
Techniques such as amino acid mutation, chemical modifications (e.g., PEGylation), and molecular conformation optimization are used to improve the stability of antibodies under various environmental conditions. These techniques help maintain the structure and function of antibodies, thereby enhancing their therapeutic effectiveness and durability.
3. Reducing Immunogenicity
Strategies such as humanization, removal of immunogenic segments, modification of antibody glycosylation, and reduction of antibody half-life are employed to decrease the immune reactions antibodies trigger in the body, improving treatment safety and efficacy.
4. Optimizing Drug Pharmacokinetics
By improving the formulation and dosage forms of antibodies, such as using excipients (e.g., surfactants and amino acids) or producing more stable constructs (e.g., protein scaffolds and bispecific molecules), the pharmacokinetic properties and stability of monoclonal antibodies are enhanced.
5. Differentiated Development Strategies
Adopting differentiated antigen epitopes, optimizing Fc effects, eliminating or reducing side effects, and enhancing the stability and solubility of antibodies to improve the drug's effectiveness, safety, and convenience of administration.
6. Exploring New Therapeutic Mechanisms and Targets
Researching and developing antibody drugs aimed at new targets, and exploring novel antibody drugs such as ADCs and bispecific antibodies to broaden therapeutic areas and enhance treatment outcomes.
Testing Content Required by Policies and Regulations
Clinical Pharmacology Considerations for Antibody-Drug Conjugates Guidance for Industry
This guidance document represents the current thinking on clinical pharmacology considerations in the development of ADCs and does not create any legal rights, nor is it binding on the public. The document is intended to assist industry and other parties involved in the development of ADCs. ADCs typically consist of a small molecule drug (payload) and an antibody or antibody fragment linked via a chemical linker. The mechanism of action of ADCs aims to deliver the drug directly to specific tissues through the antibody's targeting ability, resulting in relatively lower systemic exposure compared to traditional small molecule drugs.
1. Clinical Pharmacology Considerations
(1) Bioanalytical Mthods: All bioanalytical methods should be validated and reported according to the M10 guidelines. During the early phases of clinical trials, validated assay methods should be used to measure ADCs and their components.
(2) Dose-Exposure Response: In addition to assessing the dose-response relationship of the ADCs, exposure-response analysis for ADCs and their components should be conducted to support dose selection and adjustment.
(3) Intrinsic Factors: Assess the intrinsic factors that affect the exposure of the ADC or its components, such as renal or hepatic function impairment, pharmacogenomics, body weight, age, gender, race, and ethnicity.
(4) QTc Assessment: All ADC development projects should submit a risk assessment for QT prolongation and a QT assessment plan, focusing on the evaluation of the unconjugated payload, linker, and any pharmacologically relevant metabolites.
(5) Immunogenicity: The immunogenic response produced by ADCs should be assessed, including confirmatory evaluations for antibodies against ADCs (anti-drug antibody, ADAs).
(6) Drug-Drug Interactions (DDIs): ADC development plans should include in vitro DDI risk assessments for the unconjugated payload and relevant components of the ADC.
Points to Consider in the Manufacture and Testing of Monoclonal Antibody Products for Human Use(1997)
In the manufacturing process of mAb products, multiple factors can affect their final therapeutic effectiveness, including but not limited to the following points:
1. Cell Line Selection and Characteristics
The cell lines used for producing monoclonal antibodies need to be stable and have high production efficiency. The genetic characteristics, immunogenicity, and adaptability to specific culture conditions of the cell lines can affect the yield and quality of the antibodies.
2. Production and Purification Processes
The cell culture conditions during production (such as temperature, pH, nutrients, and oxygen supply) affect the expression and stability of the antibodies. Furthermore, the purification process must effectively remove impurities such as host cell proteins, DNA, and other potential contaminants to ensure the purity and safety of the product.
3. Protein Modifications and Glycosylation
The glycosylation patterns of mAbs can influence their immune effects and stability in the body. Inconsistencies in glycosylation during production can lead to variations in therapeutic effects.
4. Product Quality Control
Rigorous quality control procedures are crucial to ensure the consistency and compliance of each batch of product. This includes testing the product's structural integrity, specificity, potency, and safety.
5. Viral and Microbial Contamination
The risk of viral and microbial contamination must be strictly controlled during production. Viral clearance studies need to demonstrate that the purification process can effectively remove or inactivate potential contaminants.
6. Immunogenicity
mAbs may trigger immune responses in patients, which can reduce therapeutic effectiveness or cause adverse events. The immunogenicity of the product must be assessed through preclinical and clinical studies.
7. Stability and Storage Conditions
The stability of mAbs is crucial for their long-term storage and transport. Unstable antibodies may degrade, reducing their therapeutic effect.
8. Dosage and Administration Method
Appropriate dosage and administration method are essential for achieving therapeutic effects. Too low a dose may lead to poor treatment outcomes, while too high a dose could increase the risk of toxicity.
9. Patient Selection
Specific characteristics of patients, such as antigen expression levels, immune status, and disease stage, can affect the therapeutic effects of mAbs.
10. Manufacturing Changes
Any changes in the manufacturing process during product development can affect the quality and therapeutic effectiveness of the product. Therefore, rigorous comparative studies of the product are necessary to demonstrate consistency before and after changes.
Bispecific Antibody Development Programs | Guidance for Industry | Draft Guidance
1. Scientific Considerations
(1) CMC (Chemistry, Manufacturing, and Controls) Quality Considerations: Bispecific antibodies can exist in various forms, including tandem single-chain variable fragments and antibodies based on Immunoglobulin G (IgG), which have multiple additional antigen-binding domains attached. These different forms allow bispecific antibodies to be designed to match the proposed mechanisms of action and intended clinical applications.
(2) Non-Clinical Studies: Non-clinical studies are typically required to characterize the pharmacology and toxicology of bispecific antibodies. The scope of non-clinical plans is expected to be similar to that for mAbs targeting a single antigen.
(3) Clinical Pharmacology: Clinical pharmacology studies of bispecific antibodies should consider their binding to each target. It may be necessary to develop multiple assay methods to quantify the appropriate forms and consider the impact of immune responses on different structural domains.
Immunogenicity Testing of Therapeutic Protein Products — Developing and Validating Assays for Anti-Drug Antibody Detection
This document outlines recommendations for developing and validating various assays—screening, confirmatory, titer, and neutralization—to assess the immunogenicity of therapeutic protein products in clinical trials. It details key assay design elements that cover a broad range of testing aspects, specifically including:
1. Testing Strategy
Employ a multi-tiered testing approach by initially utilizing sensitive screening assays to evaluate clinical samples. Confirmatory assays are then used to verify antibody specificity, followed by further characterization through titer and neutralization assays.
2. Immunoglobulin Isotypes or Subtypes
Screening assays should detect all relevant immunoglobulin isotypes, such as IgM, IgG, and IgA.
3. Domain Specificity
For protein products with multiple functional domains, it is necessary to investigate whether ADA specifically bind to clinically relevant domains.
4. Assay Cut-Point
Establish the assay cut-point, which defines the response level at which a sample is considered positive or negative.
5. Sensitivity
The assay should be sufficiently sensitive to detect ADA before it reaches levels that might affect pharmacokinetics, pharmacodynamics, safety, or efficacy.
6. Drug Tolerance, Sensitivity, and Assay Suitability
Evaluate the assay's sensitivity in the presence of expected drug concentrations to ensure it is suitable for detecting ADA in subjects undergoing treatment.
7. Specificity
The assay should specifically detect ADAs without interference from other serum components, such as drug targets, non-specific endogenous antibodies, or the antibodies used in the assay.
8. Selectivity
The assay should be able to identify ADA specifically targeting the therapeutic protein product amidst interference from other components in the sample.
9. Precision
Assay results should be reproducible across different assay runs to ensure accuracy.
10. Reproducibility
Maintain assay reliability under typical conditions and ensure sample integrity through proper storage and handling.
11. Robustness and Sample Stability
Assays should demonstrate consistent reliability under standard operating conditions. Additionally, protocols for sample storage and handling must ensure that antibody reactivity is maintained.
12. Selection of Format
Choose an appropriate assay format based on the characteristics and requirements of the therapeutic protein product being tested.
13. Selection of Reagents
It is essential to carefully select and develop control antibodies and other reagents for use in assays, with a focus on ensuring their high quality and stability to support reliable results.
Antibody Drugs Analysis Solutions
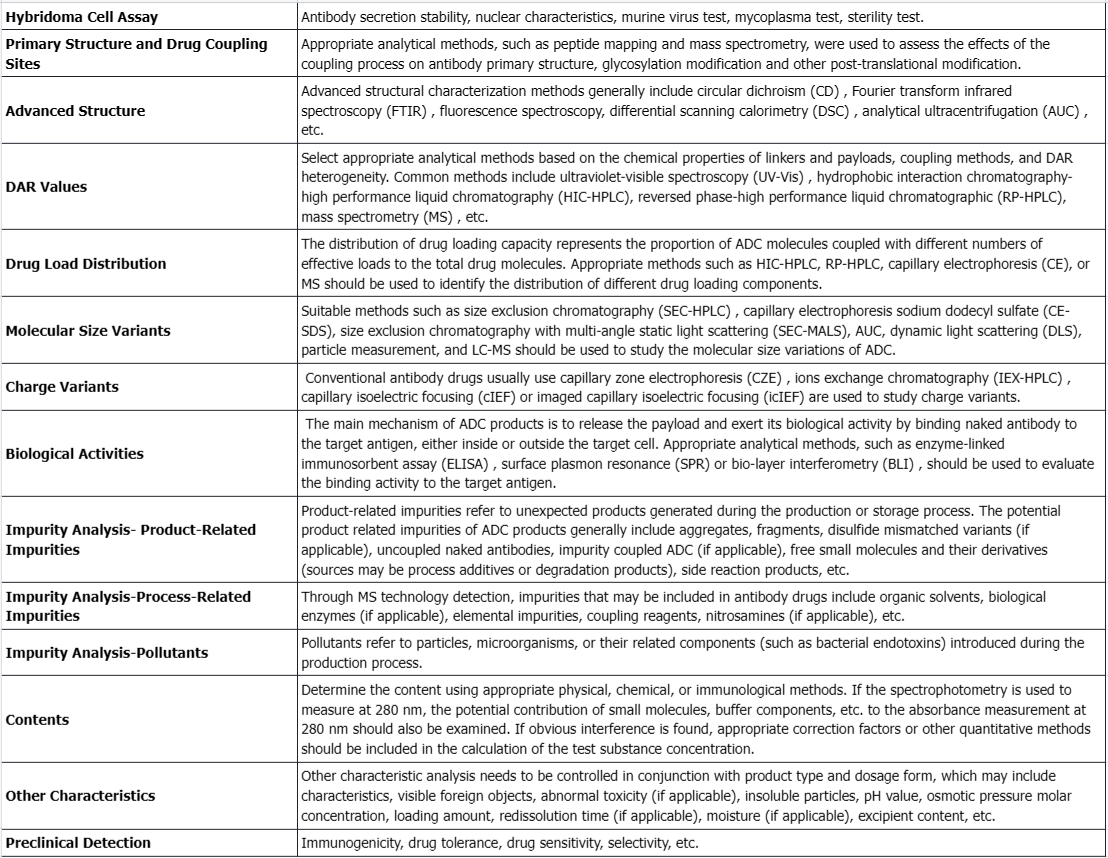
mAb intermediates, drug-linker intermediate, active pharmaceutical ingredient, and drugs recommended quality attributes for selection for comparability studies.
Service Advantages
1. Comprehensive Professional Services
Provides robust and specialized services encompassing a broad spectrum of antibody drug testing, ensuring thorough evaluation and analysis.
2. Efficient and Cost-Effective Testing
Delivers high cost-effectiveness with quick turnaround times for experiments and produces detailed and reliable testing reports.
3. Advanced Instrumentation
Equipped with a range of high-resolution mass spectrometers and other sophisticated large-scale instruments, facilitating precise and advanced testing capabilities.
Sample Results
1. Practical Approaches Using Novel Methods and Ultra-High Resolution MS to Overcome The Characterization Challenges of ADCs
Research has utilized novel methods and ultra-high resolution (UHR) MS to characterize ADCs, providing specific examples of these methods. Typical LC/MS analysis under denaturing conditions hinders the full characterization of intact 4-chain ADC, where the hinge region disulfide bonds are partially reduced. However, under non-denaturing SEC/MS conditions, such ADCs can be reliably detected intact, also known as native MS. For ADCs with acid-labile linkers, such as those used for calicheamicin conjugation, carefully choosing the mobile phase composition is crucial for retaining the intact linker-payload (LP) during LC/MS analysis. Increasing the pH of the mobile phase prevents the cleavage of unstable bonds in the linker part, resulting in the retention of the complete LP. In the intact mass analysis of ADCs, in-source fragmentation was also observed for the specific surface-accessible LP moiety coupled to the heavy chain C-terminal tag LLQGA (via transglutaminase chemistry). Optimization of other ESI source parameters, such as cone voltage, gas pressure, and ion transfer parameters, enables minimal fragmentation and optimal sensitivity. The combination of UHR MS with reversed phase-ultrahigh performance (RP-UHP) LC and the use of FabRICATOR® enzyme offers a highly resolved analysis of antibody subunit structures, facilitating rapid confirmation of integrity and conjugation level.
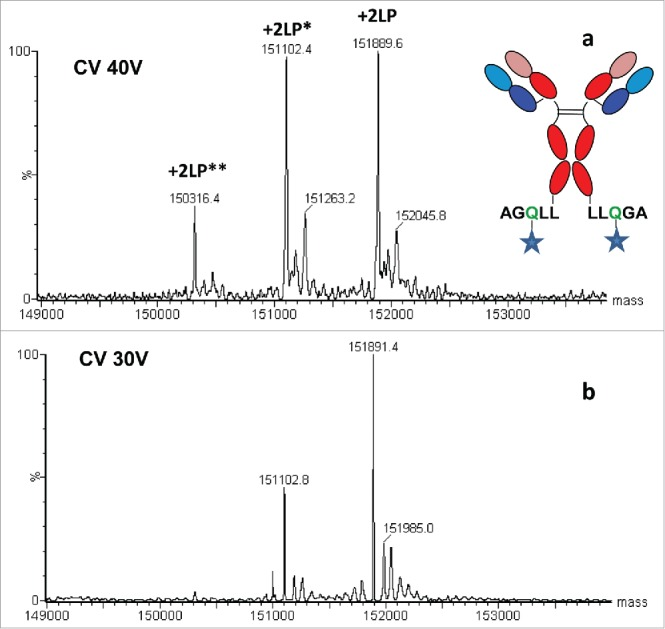
Figure 2. Mass Spectrum of Site-Specific Conjugated ADC with Surface Exposed LP [2]
2. Using Polarized Excitation Emission Matrix Spectroscopy to Analyze the Protein Conjugation Reaction in ADCs Synthesis
ADCs are a promising anti-cancer therapy, offering significant advantages over more classical treatments. The key quality attributes (CQAs) of ADCs, such as protein structure, aggregation, and DAR, influence their efficacy, stability, and toxicity. The production process exposes antibodies to various physical and chemical stresses, as well as increased aggregation due to hydrophobic drug conjugation, which can compromise antibody stability. Therefore, to maintain CQA levels, appropriate handling, production, and storage control strategies are essential. These strategies require in-process quality measurements to first identify, understand, and control variables that adversely affect ADC CQAs during manufacturing. Here, we demonstrate how polarized excitation emission matrix (pEEM) spectroscopy—a sensitive, non-destructive, and potentially rapid technique—can be used to quickly assess aggregation and DAR in a single measurement. pEEM provides multiple sources of information for protein analysis: Rayleigh scattering for identifying aggregates/particles formation, and fluorescence emission to assess chemical and structural changes caused by linker and/or small molecule drug payload attachment. Studies have utilized non-toxic ADC mimic (mAbs with a linker molecule) to demonstrate the method's effectiveness. Changes in emission caused by the linker's light absorption enable us to accurately predict DAR using fluorescence signals from the final purified product (predicted relative error [REP] 6%) and unpurified alkylated intermediates (11% REP). pEEM variations may also correlate with size (hydrodynamic radius, Rh) and aggregation content parameters obtained from DLC and SEC. For starting material and purified product samples, pEEM correlates better with Rh (R² = 0.99, 6% REP) than the total content determined by SEC (18% REP). Combining fluorescence and light scattering signals also allows for in-process size quantification (6% REP). Overall, integrating polarized measurements with EEM and Rayleigh scattering provides a single-measurement, multi-attribute testing method for ADC manufacturing.
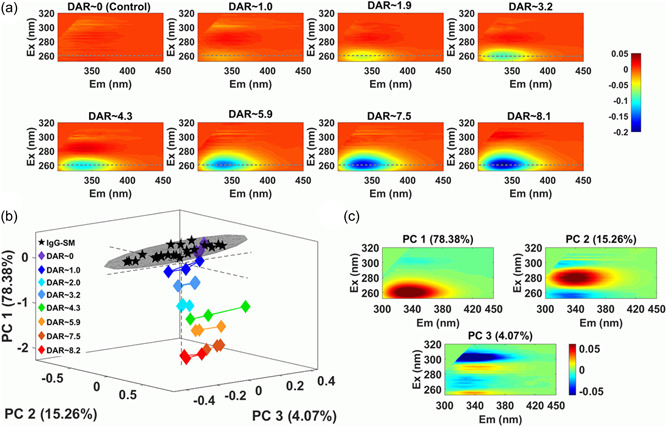
Figure 3. pEEM Measurement to Monitor the Reaction of Important mAb Joint Coupling Processes [3]
References
[1] Goulet DR, Atkins WM. Considerations for the Design of Antibody-Based Therapeutics. J Pharm Sci. 2020;109(1):74-103. doi:10.1016/j.xphs.2019.05.031
[2] Friese OV, Smith JN, Brown PW, Rouse JC. Practical approaches for overcoming challenges in heightened characterization of antibody-drug conjugates with new methodologies and ultrahigh-resolution mass spectrometry. MAbs. 2018;10(3):335-345. doi:10.1080/19420862.2018.1433973
[3] de Faria E Silva AL, Ryder AG. Analyzing protein conjugation reactions for antibody-drug conjugate synthesis using polarized excitation emission matrix spectroscopy. Biotechnol Bioeng. 2022;119(12):3432-3446. doi:10.1002/bit.28229
How to order?







