Olink Analysis Service
With the increasing focus on personalized healthcare, there is a growing demand for biomarkers for early diagnosis, prognosis, patient stratification, or monitoring treatment response. Compared with genetic biomarkers, protein biomarkers provide higher degrees of differential information. Olink proteomics is based on a unique proximity extension assay (PEA) technique that enables simultaneous quantification of multiple protein biomarkers. Olink PEA uses 2 matching antibodies for each target antigen. Each antibody pair is labeled with a unique DNA oligonucleotide barcode. When antibodies bind to the same target protein in solution, the DNA oligochain will anneal to sufficient stability to achieve DNA elongation. Then the DNA barcode is amplified and the amplicons obtained through qPCR or next-generation sequencing (NGS) is measured for absolute or relative quantification. PEA can detect protein with high sensitivity.
Olink proteomics provides high-throughput solutions for protein biomarker discovery, enabling the detection of numerous proteins in biological samples with concentration low to microscale, and its analytical methods are rigorously validated, which can provide superior specificity at multiple levels. Due to its high sensitivity and high throughput, Olink proteomics has been widely used in the fields of biomarker discovery, disease mechanism research, drug development and personalized healthcare. In clinical biomarker research, it can be used in all stages of drug development, including early detection and preclinical and clinical development.
Analysis Workflow
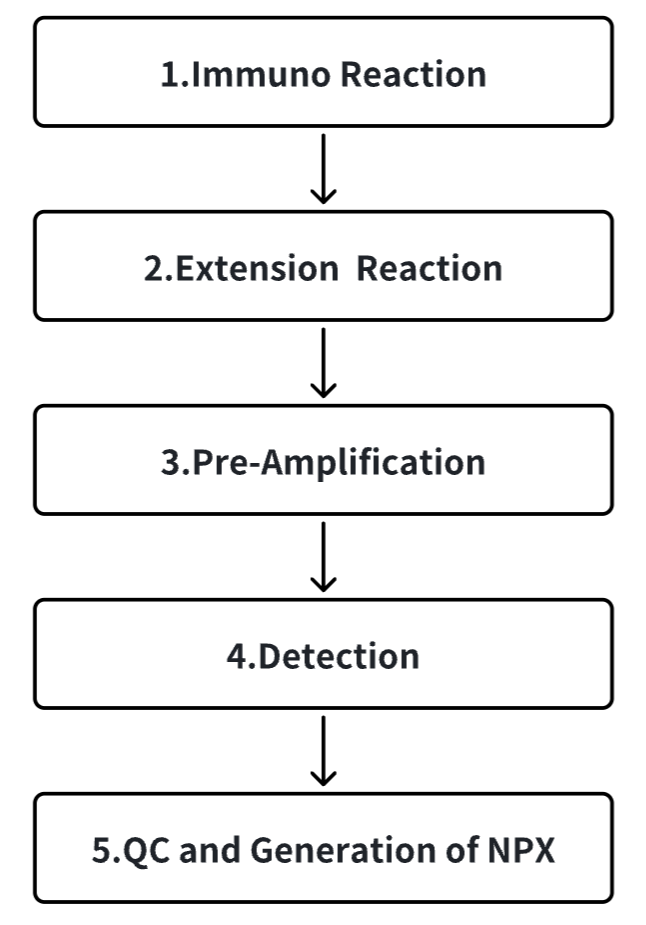
Services at MtoZ Biolabs
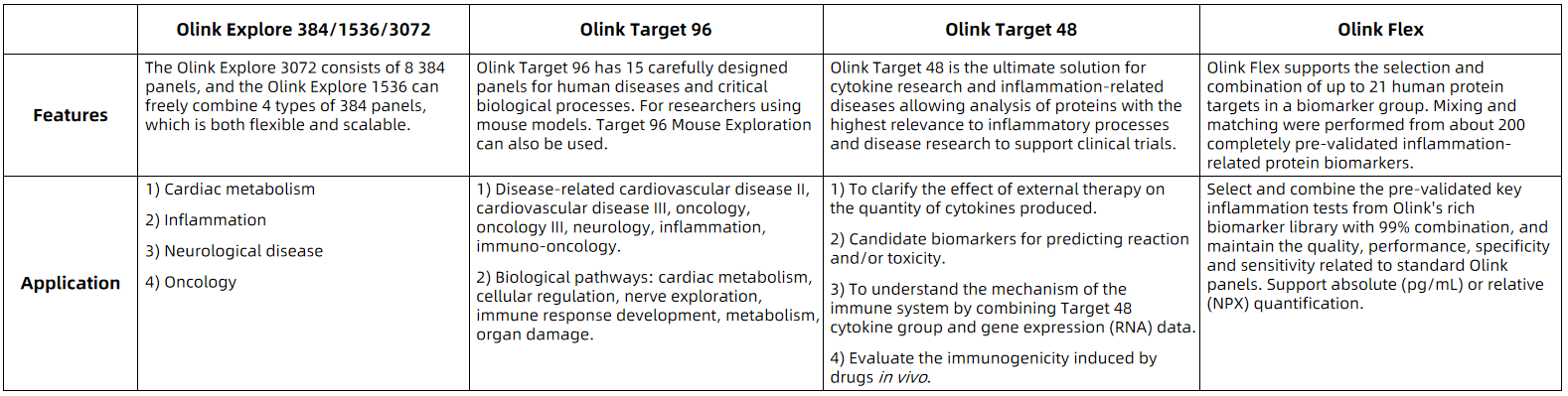
MtoZ Biolabs adopts Olink Explore384/1536/3072, Olink Target 96, Olink Target 48 and Olink Flex protein detection platform developed by Olink company. Its unique PEA technology enables high-throughput, multiplex immunoassay-PCR. Simultaneous analysis of proteins with high specificity and sensitivity only need a minimum sample concentration (as low as 1 µL). The innovative dual-recognition and DNA-coupled approach have excellent read-out specificity and enables high-throughput, rapid-throughput identification of protein biomarkers. Click to view MtoZ Biolabs detectable protein markers.
Service Advantages
1. Low Starting Volume and High Throughput
Enables accurate detection of 48 to 3,072 biomarkers from a single 1–6 µl biological sample.
2. High Sensitivity and Specificity
The unique PEA (Proximity Extension Assay) technology detects protein concentrations as low as fg/ml and identifies homologous proteins with 90% similarity.
3. Rigorous Quality Control System
Internal and external controls ensure strict accuracy in detection.
4. Diverse Sample Types
Besides conventional serum and plasma, this method also supports detection in various biological samples, including cerebrospinal fluid, cell culture media, and tissue lysates.
Applications
1. Drug Target Identification
(1) Identify protein quantitative trait loci (pQTLs) to link genetic variations, proteins, and diseases.
(2) Delve into tumor biology, immune regulation mechanism, phenotypic change, and the transition from cold tumor to hot tumor.
2. Biomarker Analysis and Screening
Analyze longitudinal biomarker data between early and late time points to identify key biomarkers related to the transition from drug sensitivity to drug resistance.
3. Protein Response Validation in Clinical Trials
(1) Predict and monitor therapeutic response, sensitivity, and immune resistance mechanism.
(2) Guide strategies to optimize response rates and identify new target pathways.
4. Exploratory Endpoints in Clinical Trials
(1) Explore the correlation between circulating protein biomarker and clinical outcome.
(2) Predict the onset and mechanisms of treatment-related toxicity and monitor cytokine release syndrome and neurotoxicity.
(3) Predict relapse and treatment progression in adjunct therapy.
Deliverables
In the results report, MtoZ Biolabs will provide you with a detailed results report, which includes:
1. Experimental Procedures
2. Absolute Quantification Data (pg/mL) (Limited to Olink Target 48)
3. Relative Quantification Data: Expressed in Normalized Protein Expression (NPX) Format
4. Data Visualization: Includes Principal Component Analysis and Heatmaps to Visualize Sample Distribution and Protein Expression
5. Statistical Analysis: Includes Differential Protein Expression Analysis
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Quantitative Proteomics Analysis Service | Biopharmaceutical
How to order?







