Biopharmaceuticals Characterization Service
Biopharmaceuticals refer to drugs produced using biotechnological methods, including monoclonal antibodies, recombinant proteins, vaccines, and gene therapy products. These drugs are typically derived from living organisms and exhibit complex molecular structures and biological functions. This service utilizes advanced technical platforms to comprehensively analyze the physical, chemical, and biological properties of biopharmaceutical products, aiming to provide in-depth understanding of their molecular structures, functional characteristics, and in vivo behavior.
Biopharmaceuticals characterization service is widely applied in the research, quality control, and production of biopharmaceuticals, playing a crucial role in the development and manufacturing of monoclonal antibodies, recombinant proteins, vaccines, and gene therapy products. Accurate characterization ensures the quality, purity, and safety of these drugs, offering strong data support for both preclinical and clinical research.
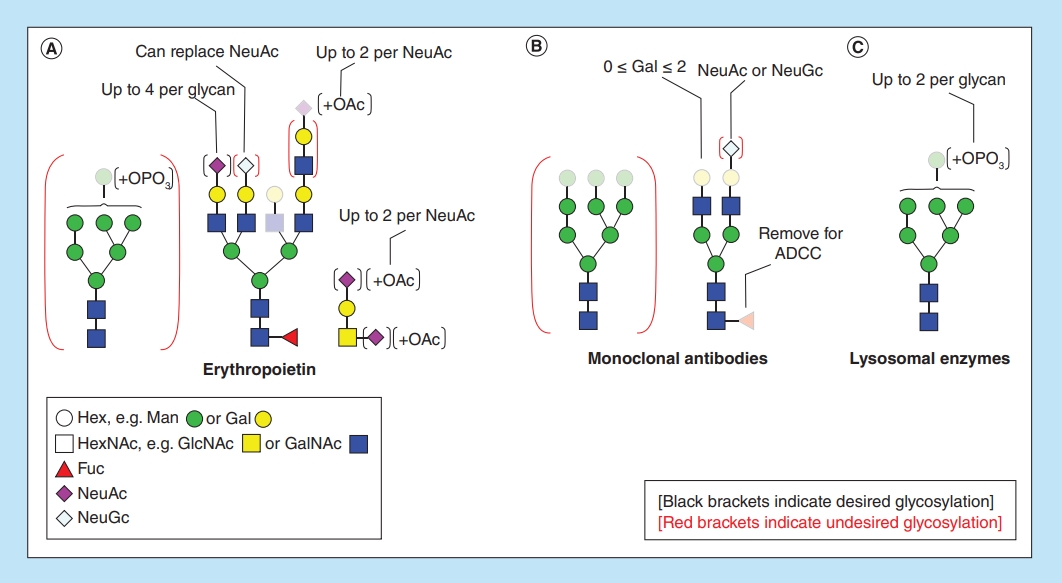
Oh, M J. et al. Bioanalysis, 2016.
Figure 1. Archetypal N- and O-glycans Found on Biotherapeutic.
Services at MtoZ Biolabs
Based on advanced technologies and analytical platforms, MtoZ Biolabs has launched biopharmaceuticals characterization service focusing on the comprehensive profiling of biopharmaceutical products. This service enables precise analysis of molecular weight, post-translational modifications, amino acid sequences, and stability of biologics. Clients receive detailed qualitative and quantitative data to support drug development, quality control, and process optimization. MtoZ Biolabs offers, but is not limited to, the following services:
1. Quantitative Proteomics Analysis Service | Biopharmaceutical
This service utilizes high-resolution mass spectrometry to perform quantitative proteomic analysis of biologics, accurately measuring protein components and changes. It delivers critical data for drug optimization and quality control.
2. Single-Cell Analysis Service
Powered by high-throughput single-cell platforms, this service explores the effects of biopharmaceuticals at the single-cell level. It reveals cellular responses and mechanisms of action, facilitating precision therapeutics and optimized drug screening.
Service Advantages
1. High Efficiency and High Throughput
Utilizing high-throughput technologies to rapidly process large volumes of samples, improve data acquisition efficiency, shorten research timelines, and support accelerated drug development and clinical studies.
2. High-Precision Data Analysis
Providing highly accurate qualitative and quantitative data on drug components through advanced mass spectrometry and quantitative proteomics analysis to ensure the reliability and precision of research outcomes.
3. Customized Solutions
Delivering tailored analytical plans based on specific customer requirements to ensure that the unique characteristics of each biopharmaceutical product are accurately evaluated.
4. One-Stop Service
Offering end-to-end services from sample testing to biological data interpretation, covering all stages of drug development to provide comprehensive data support for pharmaceutical research and optimization.
Applications
1. Biopharmaceutical Development and Functional Optimization
This service supports the structural and functional analysis of recombinant proteins, antibody drugs, and more, facilitating targeted design and molecular optimization.
2. Drug Batch Consistency Evaluation
The biopharmaceuticals characterization service can be used to compare protein characteristics across different production batches, ensuring product consistency and quality stability.
3. Impurity and Modification Component Detection
By accurately identifying trace impurities, aggregates, or post-translational modifications in drug products, this service ensures drug purity and safety.
4. Stability Studies
The biopharmaceuticals characterization service can be used to assess the structural integrity and activity retention of drugs under storage, transport, and various conditions, helping to optimize production and preservation processes.
FAQ
Q1: Can Post-Translational Modifications (PTMs) Be Analyzed?
A1: Yes. We can accurately identify and quantify various PTMs such as glycosylation, phosphorylation, acetylation, oxidation, and deacetylation. This provides valuable data for studying drug function mechanisms and stability.
Q2: How Does this Service Support Protein Stability and Quality Control?
A2: By analyzing characteristics such as protein aggregation, purity, and post-translational modifications, the Biopharmaceuticals Characterization Service provides critical insights into protein stability, degradation pathways, and product quality control, helping ensure drug consistency and quality.
Related Services
Quantitative Proteomics Analysis Service | Biopharmaceutical
How to order?







