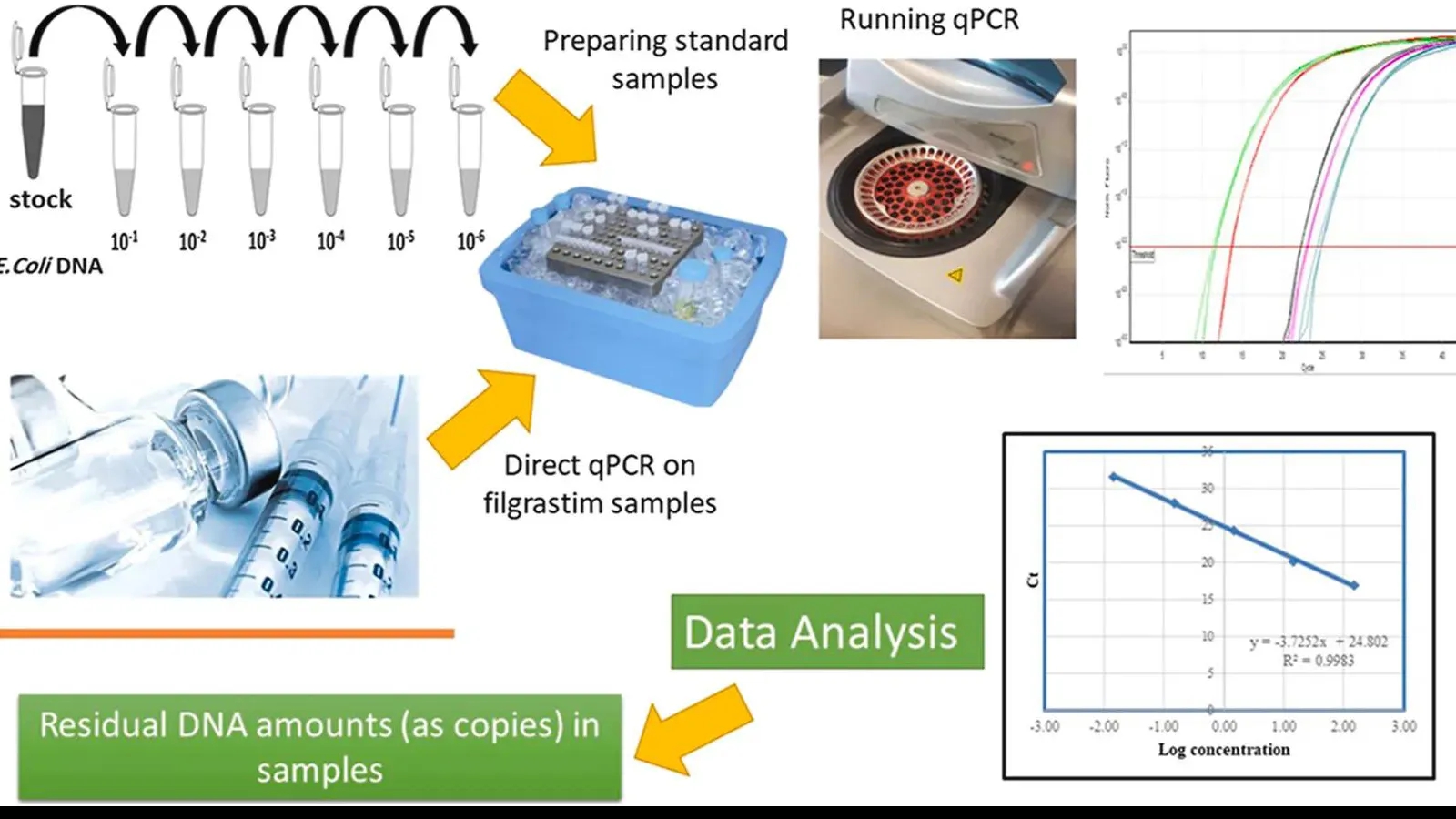
Preclinical Drug Development
Preclinical drug development marks a critical phase in the drug development pipeline, bridging laboratory discoveries with human clinical trials. This stage is designed to thoroughly evaluate a candidate compound’s safety, efficacy, pharmacokinetics (PK), and potential toxicity before clinical testing. The outcomes of preclinical studies play a decisive role in enabling Investigational New Drug (IND) applications and advancing into clinical trials.
MtoZ Biolabs offers end-to-end Preclinical Drug Development Services through our integrated research platforms and expert scientific teams. From basic research and target validation to lead compound optimization and IND-enabling studies, we deliver comprehensive support to help transform promising molecules into clinical-stage drug candidates efficiently and effectively.
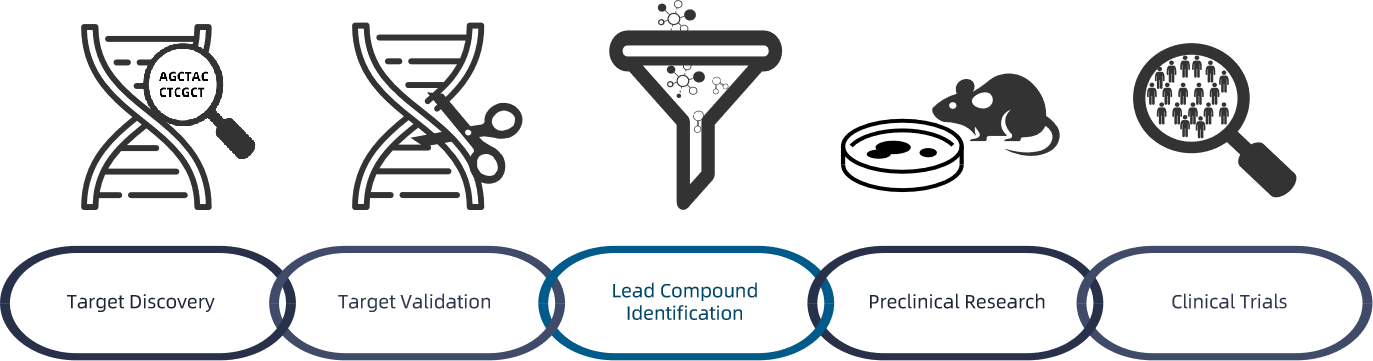
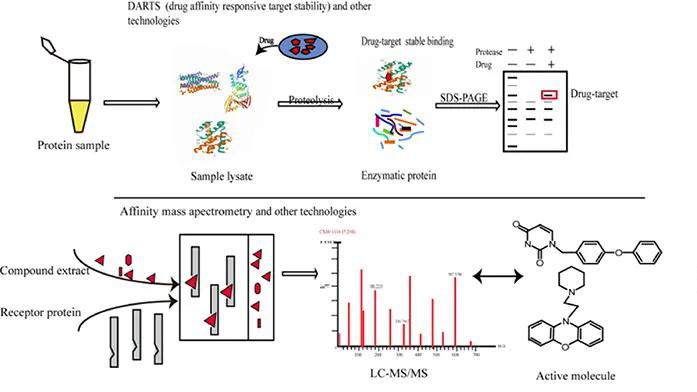
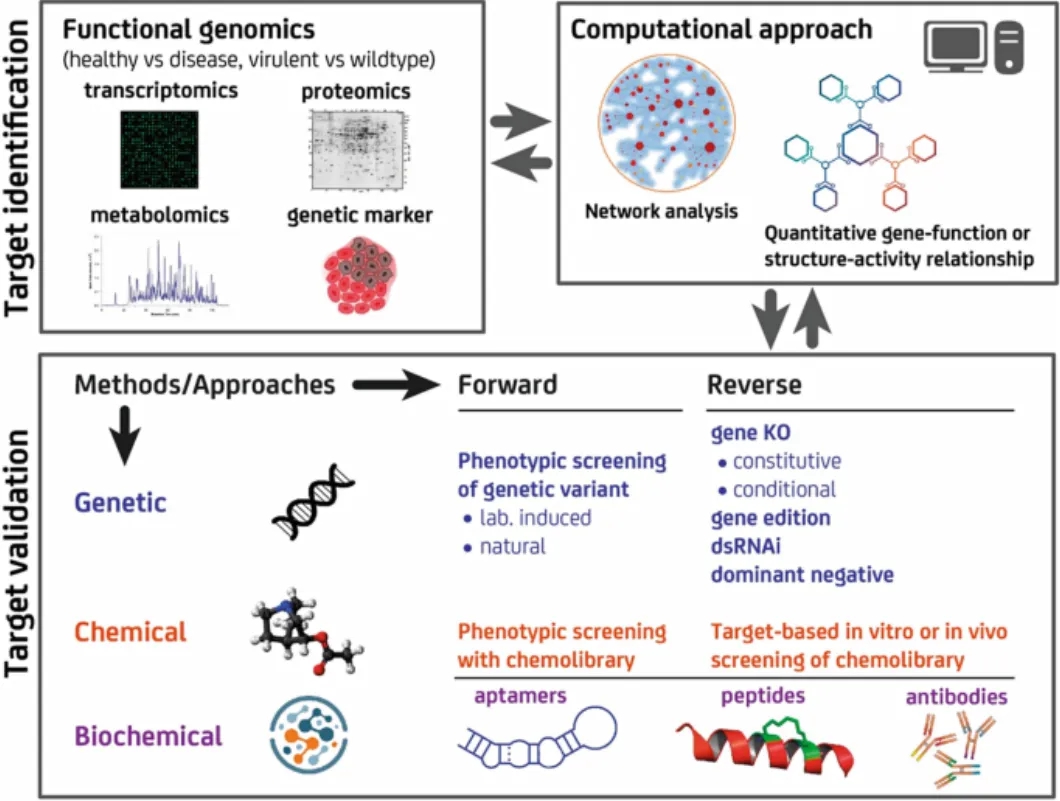
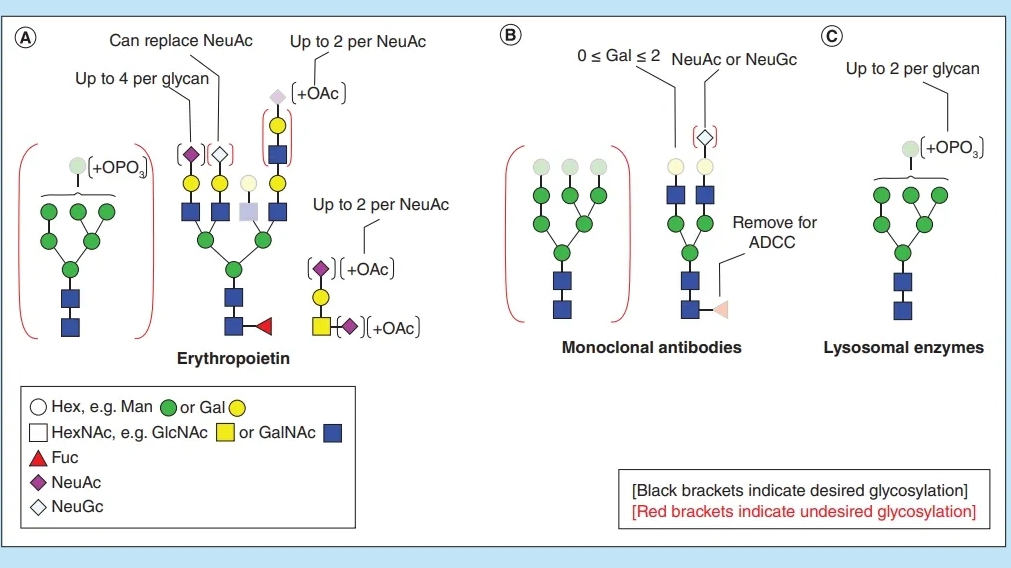
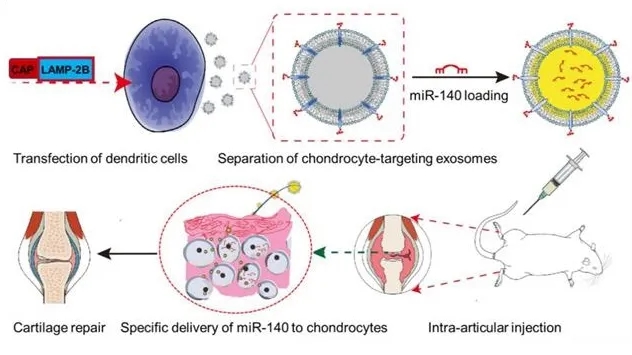
Figure 1. Preclinica Drug Development Process
Services at MtoZ Biolabs
Service Advantages
✅ Advanced Analysis Platform
We leverage state-of-the-art platforms including LC-MS/MS, Orbitrap mass spectrometry, digital PCR, flow cytometry, and multi-omics profiling to support mechanism-of-action studies and biomarker discovery.
✅ Comprehensive Disease Model Portfolio
Our standardized in vivo models cover a wide range of disease areas, including oncology, autoimmunity, infectious diseases, and metabolic disorders—enabling robust and translatable efficacy evaluations.
✅ Modular and Integrated Solutions
Whether you need a stand-alone study or a full IND-enabling package, our flexible service models adapt to your project timeline and budget, supporting each stage of preclinical development.
✅ One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
Sample Submission Suggestions
We accept various candidate drug types, including small molecules, biologics (e.g., antibodies, recombinant proteins, oligonucleotides), and cell/gene therapy materials. All samples should be submitted with detailed batch and concentration information and must comply with relevant transportation and animal research regulations.
Please contact us to receive a tailored sample submission and shipping guideline.
FAQ
Q1: What animal models do you offer for PK and efficacy studies?
We support a broad spectrum of species including mice, rats, guinea pigs, rabbits, dogs, and non-human primates. Customized models such as humanized mice, PDX models, or disease-specific systems are also available to match various mechanisms and indications.
What Could be Included in the Report?
· Full reports for efficacy, toxicology, PK, CMC, and analytical methods
· GLP-compliant or non-GLP data packages
· Raw data, statistical outputs, and annotated charts
· Biomarker discovery results and mechanism-of-action models (when applicable)
· Project summary, regulatory strategy suggestions, and structured registration documents (optional)
At MtoZ Biolabs, we are committed to accelerating drug discovery through high-quality, efficient, and regulatory-aligned preclinical research. Our Preclinical Drug Development Services span new target discovery, efficacy validation, safety assessments, and PK studies—ensuring your candidate drug is fully prepared for clinical translation.
Contact us today to discuss your project and receive a customized preclinical development plan.
Related Services
Biopharmaceutical Research Services
Biological Products Analysis Service
MtoZ Biolabs, an integrated Chromatography and Mass Spectrometry (MS) Services Provider, provides advanced proteomics, metabolomics, and biopharmaceutical analysis services to researchers in biochemistry, biotechnology, and biopharmaceutical fields. Our ultimate aim is to provide more rapid, high-throughput, and cost-effective analysis, with exceptional data quality and minimal sample consumption. Free project evaluation, welcome to learn more details!
How to order?