Medical Device Analysis Service
- Quality control and consistency evaluation of medical injectable fillers (e.g., hyaluronic acid and collagen-based injectables)
- Component identification and safety assessment of regenerative biomaterials (e.g., wound dressings and tissue engineering scaffolds)
- Raw material quality evaluation and risk screening for suppliers
Medical Device Analysis Service refers to a comprehensive suite of services that utilize high-precision chemical analysis and structural characterization techniques to perform component identification, quality control, stability testing, and compliance evaluation of various medical devices and their raw materials. This service is designed to ensure that products meet regulatory standards and safety performance requirements, supporting companies through product development, manufacturing, and regulatory review stages.
With the widespread application of medical devices in clinical diagnostics, tissue engineering, and aesthetic restoration, product safety, functionality, and stability have become key concerns for both regulatory agencies and manufacturers. This is especially critical for Class III medical devices intended for implantation, injection, or direct contact with human tissue, where systematic analysis of raw materials, structural composition, biocompatibility, and potential risks is essential.
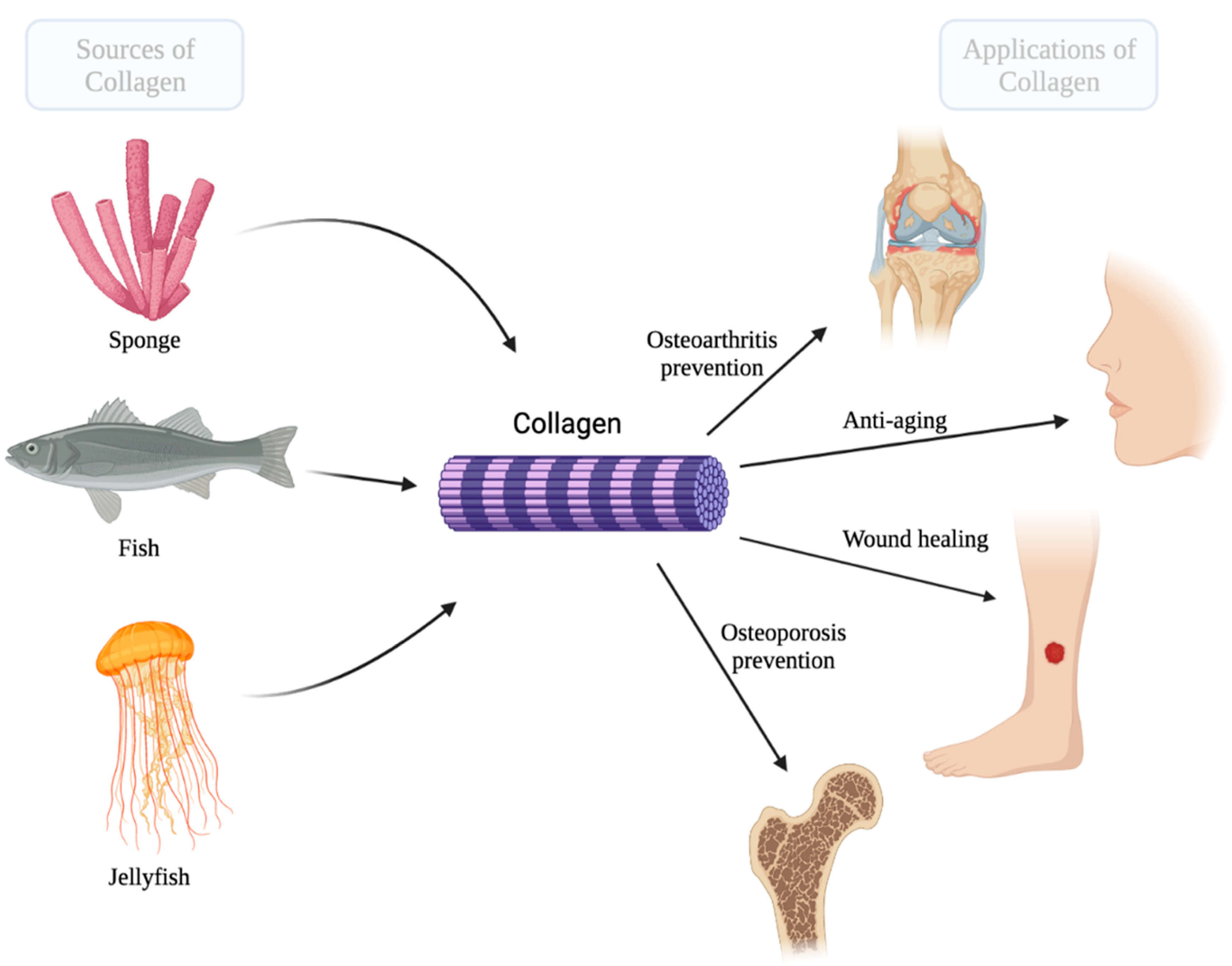
Geahchan S. et al. Mar Drugs. 2022.
Figure 1. The marine sources of collagen and its main biomedical applications.
Services at MtoZ Biolabs
Leveraging advanced analytical platforms, MtoZ Biolabs offers Medical Device Analysis Service covering key categories such as structural biomaterials, injectable viscoelastic fillers, and Class III medical products. Our services support qualitative confirmation, impurity detection, degradation product analysis, and quality consistency evaluation. The main offerings include the following:
This service targets medical-grade collagen materials, providing molecular structure confirmation, biochemical characterization, purity and residue analysis, and degradation product evaluation. It is suitable for testing both raw materials and final products used in applications such as skin repair membranes, tissue regeneration scaffolds, and injectable fillers.
Hyaluronic Acid Quality Control Testing Service
We provide accurate and platform-diverse analytical services for quality control of hyaluronic acid (HA)-containing raw materials and finished products. Additionally, we offer method development and validation, stability testing under controlled temperature and humidity, as well as extractables and leachables studies.
Medical Device III Special Analysis Service
Focusing on biomaterials like type I collagen, we provide comprehensive quality control solutions, encompassing raw material purity, endotoxin and pathogen removal, residual HCP/DNA, collagen quantification, and hydroxyproline modification identification, to biological function and robustness verification, in accordance with regulatory requirements. We also assist with quality assessment and regulatory compliance reporting for Class III medical devices and tissue engineering products.
Service Advantages
Advanced Analysis Platform: MtoZ Biolabs established an advanced Medical Device Analysis Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Multi-Platform Integrated Analysis: Combining LC-MS/MS, GC-MS, FTIR, GPC, DSC, NMR, and other instruments, we enable multi-scale analysis ranging from trace residues to macromolecular structures.
High-Sensitivity Impurity Detection: Capable of detecting impurities and crosslinking agent residues at ppm levels, meeting the stringent quality standards required for implantable and injectable products.
Expert Technical Team: Our team has extensive expertise in biomaterials such as collagen and hyaluronic acid, delivering accurate and tailored evaluation strategies.
Regulatory-Compliant Quality Standards: All testing is aligned with relevant regulatory guidelines and methods, ensuring that results are meaningful for compliance and product registration.
Customized Testing Strategies: We provide personalized analytical plans based on device category, material properties and registration requirements, supporting multi-stage needs from early research screening to final regulatory submission.
Applications
Applications of the Medical Device Analysis Service include but are not limited to:
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment (project-dependent)
4. Data Analysis, Preprocessing, and Estimation (project-dependent)
5. Bioinformatics Analysis
6. Raw Data Files
Related Services
How to order?







