Biopharmaceutical Peptide Mapping Analysis Service
In the biopharmaceutical field, peptide mapping is a pivotal technology for assuring drug safety and effectiveness. It conducts comprehensive sequence analysis of drug proteins, providing precise structural insights including amino acid composition, post-translational modifications, and potential variants. Peptide mapping is essential for confirming biosimilarity, refining manufacturing processes, and enhancing drug quality. Efficient peptide mapping allows biopharmaceutical firms to maintain product structural consistency, meeting rigorous international regulatory standards.
MtoZ Biolab offers in-depth peptide mapping services utilizing state-of-the-art liquid chromatography-tandem mass spectrometry (LC-MS/MS), complemented by expert proteolytic processing. This method effectively digests proteins, precisely measures each peptide, and ensures accurate, reproducible results.
Analysis Workflow
1. Sample Preparation
Target proteins undergo selective reduction and alkylation to ensure peptide stability and proper folding.
2. Enzymatic Digestion
Various proteases (e.g., trypsin, chymotrypsin, ASP-N) are employed for accurate cleavage, enhancing analytical coverage and precision.
3. LC-MS/MS Analysis
Advanced mass spectrometry deeply analyzes treated samples.
4. Data Verification
Experimental data are compared with theoretical values to confirm analysis reliability.
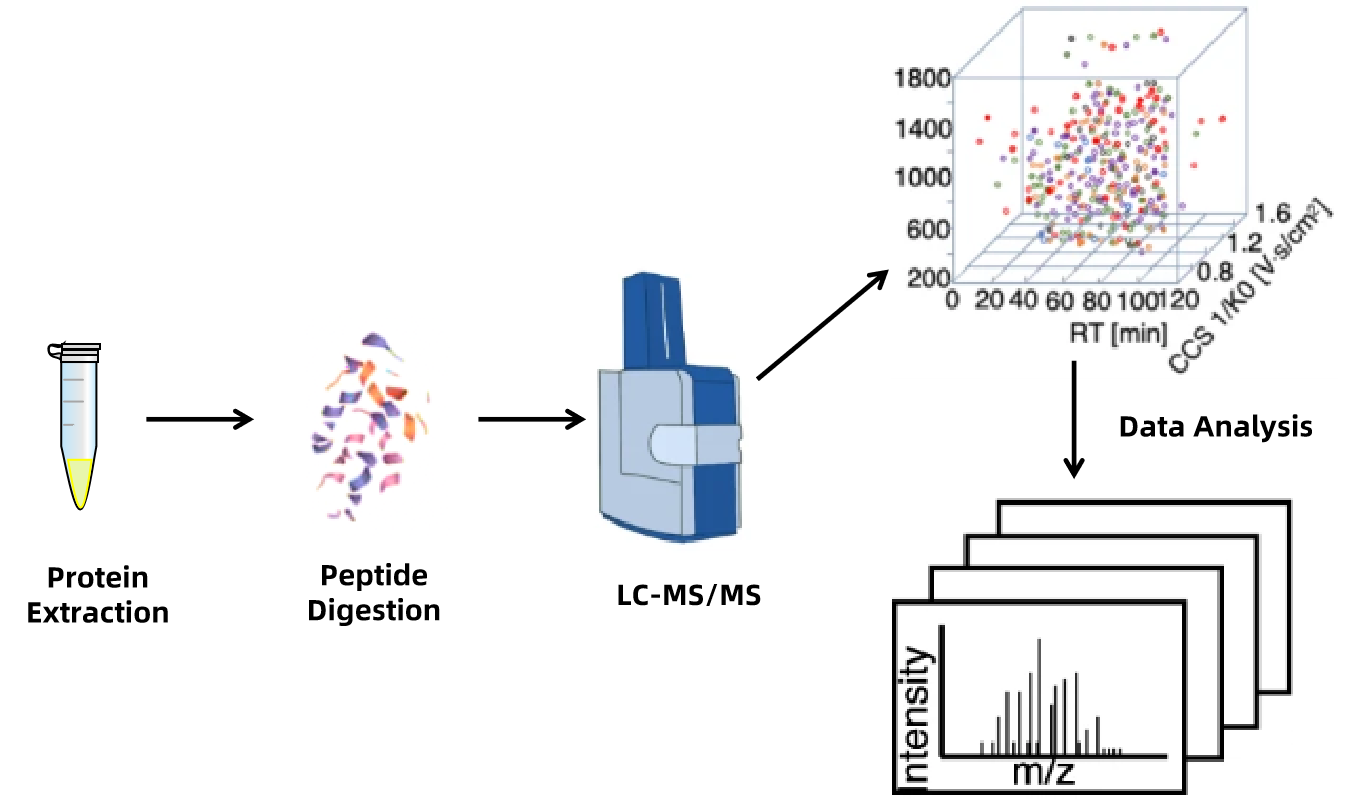
Figure 1. Analysis Workflow of Peptide Mapping
Service Advantages
Sensitivity and Precision: Detects minute protein quantities and rare post-translational modifications.
Applicability: Effective for diverse complex biological specimens.
Data Reproducibility: Guarantees consistent analytical results across and within batches.
Sample Submission Requirements
Samples must be transported under cold chain conditions with sufficient protein content to guarantee analysis precision and efficacy. Sample purity and concentration critically impact the analysis quality.
Applications
Structural Characterization: Verifies protein primary structures and evaluates post-translational modifications.
Quality Control: Tracks protein quality variations during production to ensure batch consistency.
Impurity Analysis: Identifies and quantifies product-related impurities such as deamidation, oxidants, and degradation products.
How to order?







