A Powerful Tool for Small-Molecule Drug Target Research: Thermal Proteomic Profiling (TPP)
Thermal Proteomic Profiling (TPP) is a high-throughput and unbiased proteomic screening method that integrates thermal stability analysis with mass spectrometry. The core principle of TPP is that drug-protein interactions influence protein thermal stability, leading to increased stability of drug-bound proteins upon heating. Monitoring changes in protein thermal stability provides valuable insights into ligand binding and represents a crucial step in drug target discovery. When cells or cell lysates are heated to specific temperatures, proteins denature and gradually precipitate. However, proteins bound to small-molecule drugs exhibit enhanced thermal stability, resulting in reduced denaturation under identical thermal conditions. By comparing protein melting curves (Tm) and their variations (ΔTm) across different temperatures, TPP enables proteome-wide identification of potential drug targets. Subsequent pathway enrichment analysis and experimental validation can then pinpoint key targets. TPP has been widely employed in drug target discovery, mechanism elucidation, and indication expansion, with particular advantages in uncovering multi-target effects of small-molecule drugs.
Cellular Thermal Shift Assay (CETSA) is a foundational thermal stability analysis technique that reveals small-molecule drug-protein interactions by assessing protein stability across varying temperatures. Traditionally, CETSA employed Western blotting (WB) for protein detection. Although WB offers high specificity, its limited throughput restricts its application primarily to target validation stages. With advancements in mass spectrometry-based quantitative proteomics, CETSA evolved into TPP, combining thermal stability analysis with mass spectrometry quantification. This integration provides a robust platform for comprehensive drug target analysis, significantly enhancing both efficiency and accuracy in drug target screening. In the increasingly intricate landscape of small-molecule drug research, MtoZ Biolabs offers customized TPP services drawing on its extensive expertise in proteomics and cutting-edge mass spectrometry platforms. These services support researchers in advancing novel drug discovery and detailed drug target characterization.
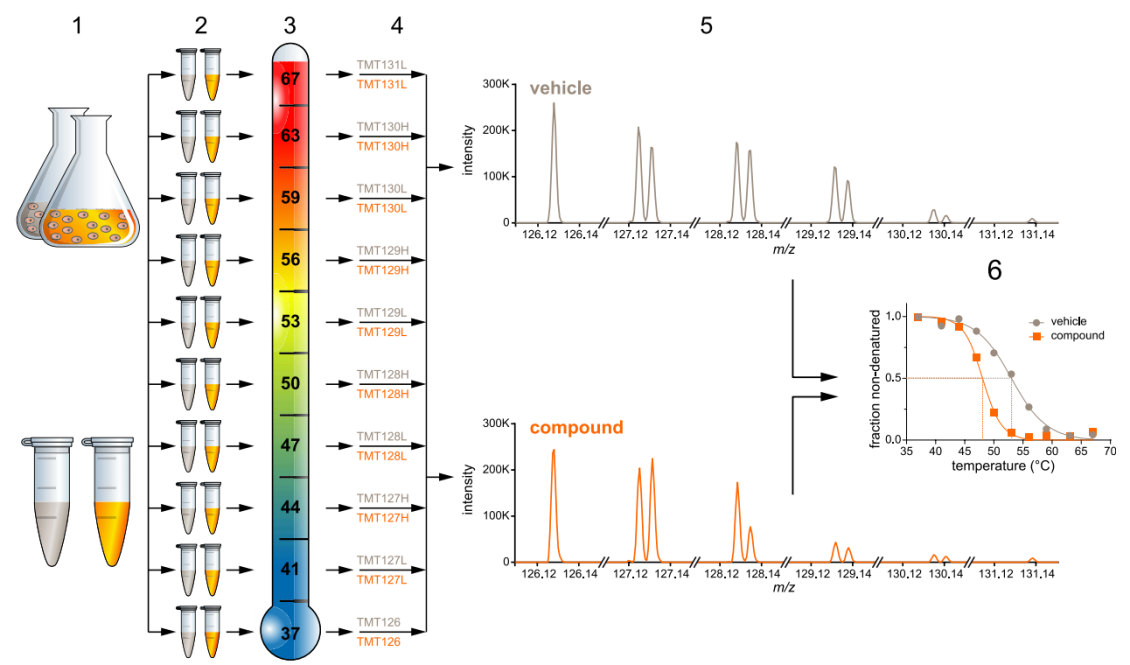
Figure 1. Analysis Workflow of TPP (Savitski et al., 2014)
Application Reference 1
Title: Lactate regulates cell cycle by remodelling the anaphase promoting complex
Journal: Nature
Impact Factor: 50.5
Year: 2023
Research Content: Lactate accumulates abundantly in rapidly proliferating cells due to enhanced glycolysis required for cellular proliferation. However, whether accumulated lactate directly influences proliferation remains unclear. Using a proteome-wide approach, researchers systematically evaluated the thermal stability of over 3,900 proteins under varying lactate concentrations using TPP. Among these, UBE2C exhibited the most pronounced lactate-dependent thermal stability shift. Further investigation revealed a regulatory mechanism wherein lactate remodels the anaphase-promoting complex (APC/C) by directly inhibiting the SUMO protease SENP1. Lactate achieves this by binding to zinc at SENP1’s active site, leading to stabilized SUMOylation of two residues on APC4. This stabilization promotes UBE2C binding to APC/C, thereby facilitating precise timing of cyclin degradation and mitotic exit in proliferating cells. This mechanism activates when lactate levels peak during mitotic entry. Conversely, sustained lactate accumulation disrupts APC/C regulation, allowing cells to bypass antimitotic treatments through mitotic slippage.
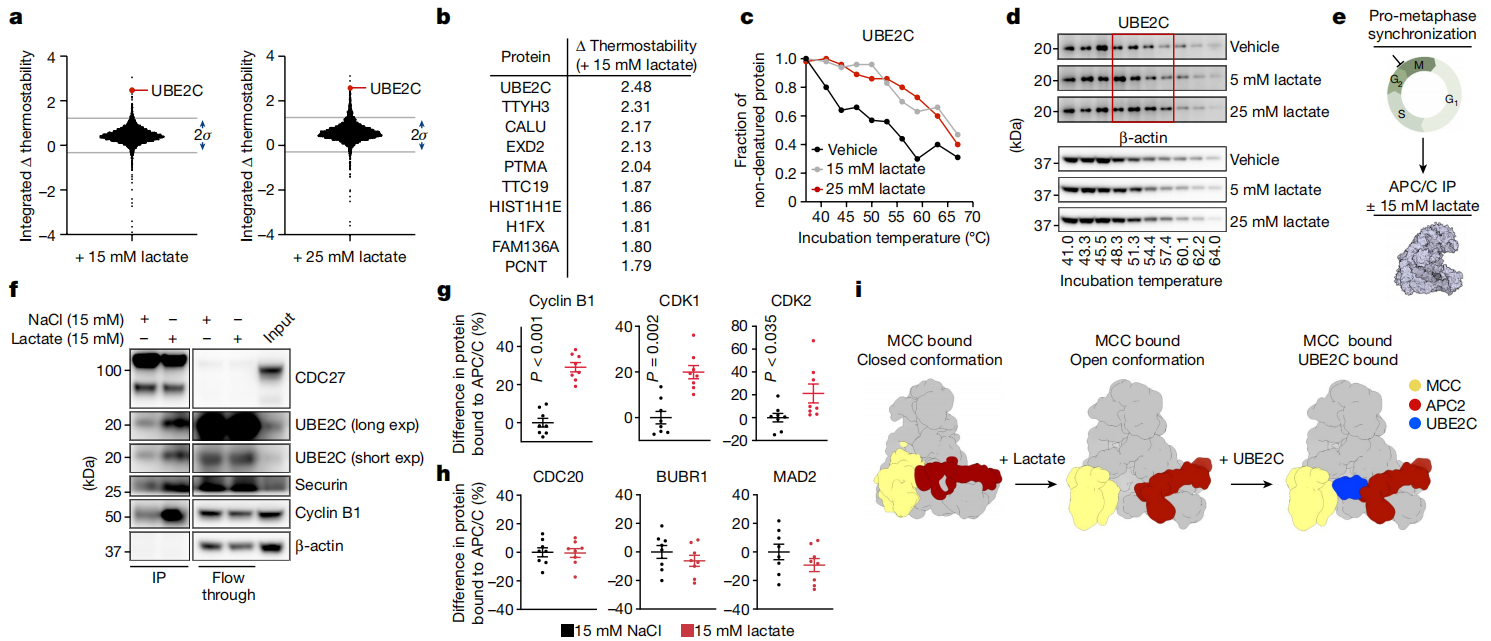
Figure 2. Lactate-Mediated Regulation of APC/C Protein Interactions
Application Reference 2
Title: Selective activation of PFKL suppresses the phagocytic oxidative burst
Journal: Cell
Impact Factor: 45.5
Year: 2021
Research Summary:
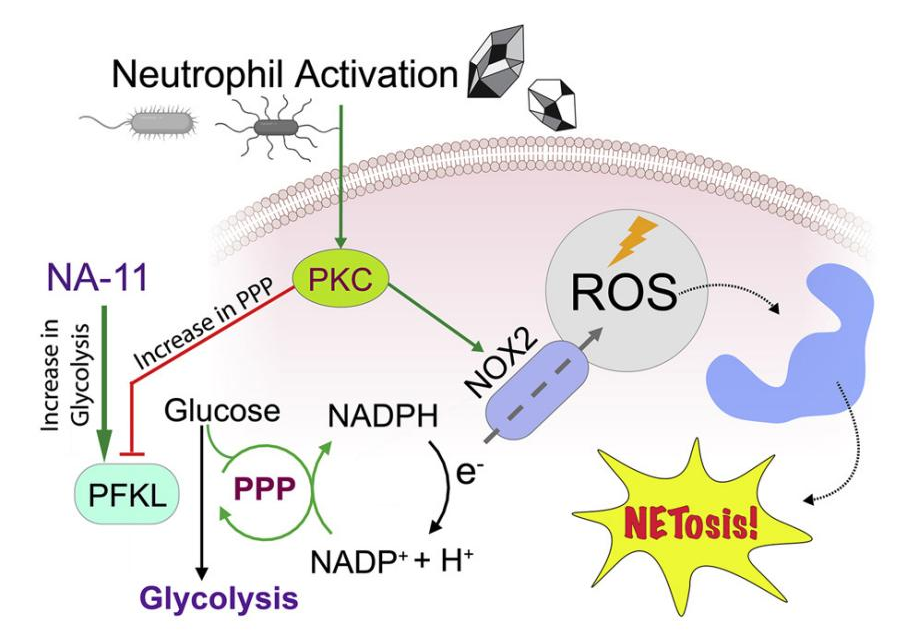
Figure 3.
Research Content: In neutrophils, NADPH derived from the pentose phosphate pathway fuels NADPH oxidase NOX2, producing reactive oxygen species (ROS) to eliminate invading pathogens. Excessive NOX2 activity, however, can exacerbate inflammatory conditions such as acute respiratory distress syndrome (ARDS). Using two unbiased chemical proteomics strategies (TPP+PISA), researchers demonstrated that the small molecule LDC7559, and its more potent analog NA-11, suppress NOX2-dependent ROS bursts by selectively activating the glycolytic enzyme phosphofructokinase-1 liver-type (PFKL). This activation reduces flux through the pentose phosphate pathway. Neutrophils treated with NA-11 exhibited reduced NOX2-dependent outputs, including neutrophil extracellular trap (NETosis) formation and tissue damage. Structural analysis confirmed that NA-11 binds specifically to the AMP/ADP allosteric activation site of PFKL, explaining its lack of activation in platelet-type (PFKP) and muscle-type (PFKM) isoforms. These findings position NA-11 as a selective chemical tool for modulating PFKL activity in immune cells.
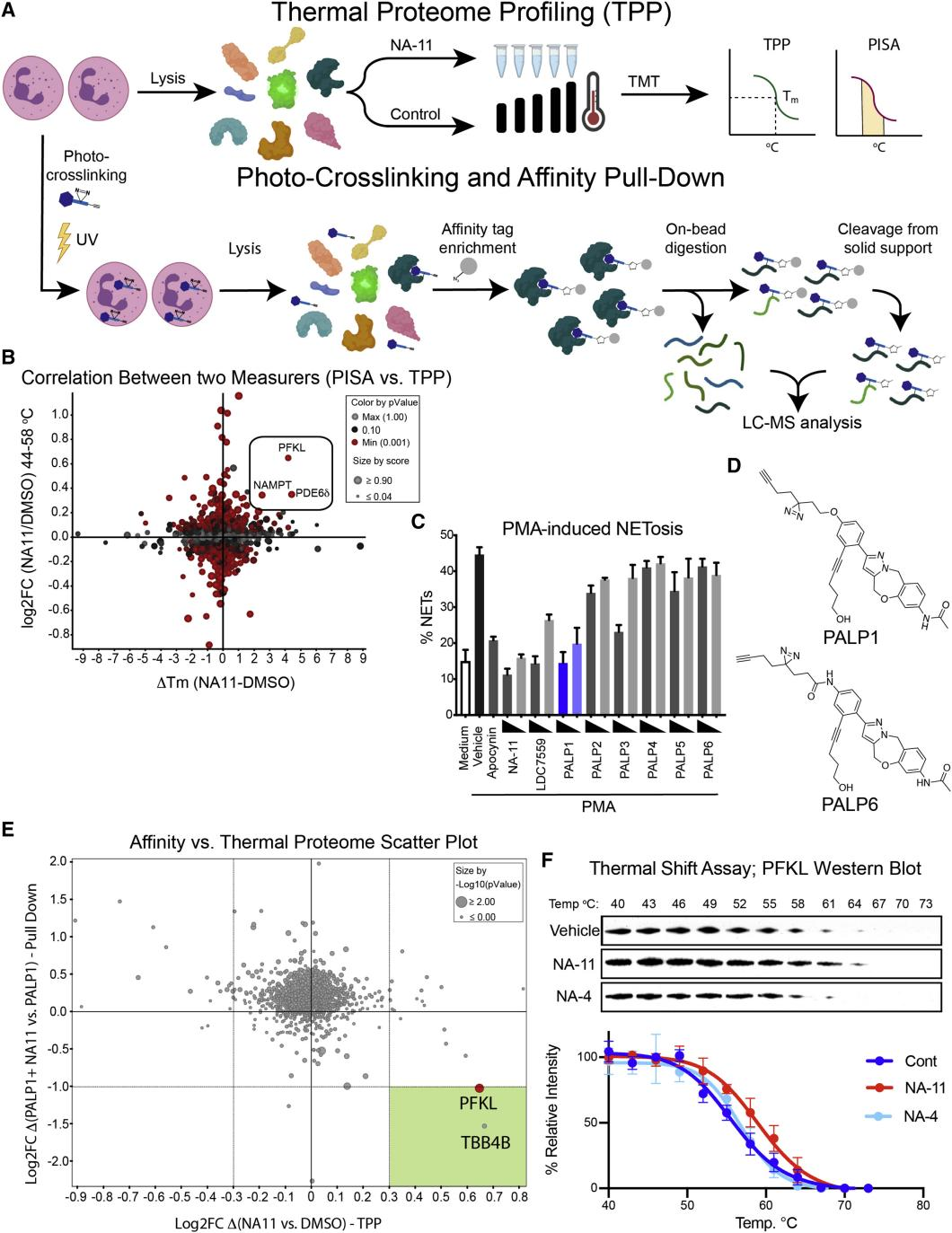
Figure 4. Global Interaction and Specific Target Analysis of NA-11 via Chemical Proteomics
Application Reference 3
Title: Thermal proteome profiling reveals fructose-1,6-bisphosphate as a phosphate donor to activate phosphoglycerate mutase 1
Journal: Nature Communications
Impact Factor: 14.7
Year: 2024
Research Content: A deeper understanding of glycolysis-protein interactions offers critical insights into glycolytic reprogramming in health and disease. Despite significant progress in studying individual interactions, a proteome-wide perspective remains challenging. Using TPP, researchers analyzed fructose-1,6-bisphosphate (FBP) interaction networks and discovered its role as a phosphate donor activating phosphoglycerate mutase 1 (PGAM1). At the molecular level, FBP transfers phosphate groups (C1-O-phosphate or C6-O-phosphate) to the catalytic histidine residue of PGAM1, forming a 3-phosphohistidine (3-pHis) modification. Structural-activity relationship analysis revealed potential inhibitors targeting PGAM1, which may suppress cancer cell proliferation. This study highlighted FBP's unique covalent signaling mechanism through histidine phosphorylation, supporting the Warburg effect and guiding the development of pharmacological tools targeting glycolysis.
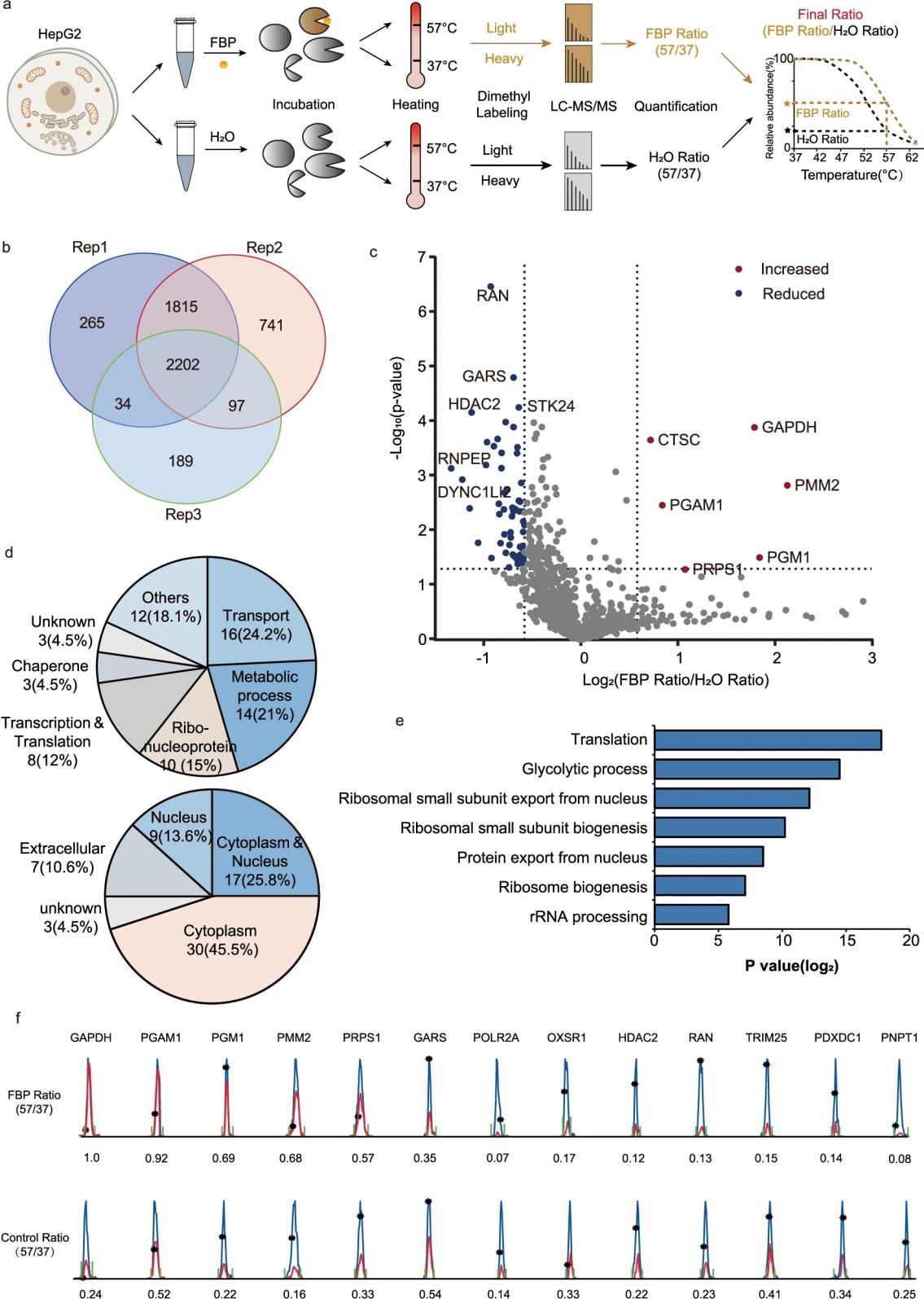
Figure 5. Thermal Proteomic Profiling of FBP-Protein Interactions
With the expanding application of TPP in small-molecule drug target research, studies on target screening, drug repurposing, and novel drug discovery have reached new heights. Through high-throughput and highly sensitive proteomic analysis, TPP efficiently identifies potential drug targets while offering robust data for mechanistic insights.
In TPP services, MtoZ Biolabs leverages its well-established experimental workflows and customizable solutions to meet diverse research needs. Partnering with MtoZ Biolabs ensures collaboration with industry-leading experts, accelerating innovation in drug discovery and development. For more information on TPP analysis services, feel free to contact our technical support team.
References
[1] Mikhail M. Savitski et al. Tracking cancer drugs in living cells by thermal profiling of the proteome.Science346,1255784(2014). DOI:10.1126/science.1255784.
[2] Liu, W., Wang, Y., Bozi, L.H.M. et al. Lactate regulates cell cycle by remodelling the anaphase promoting complex. Nature 616, 790–797 (2023). https://doi.org/10.1038/s41586-023-05939-3.
[3] Amara, Neri., et al. Selective activation of PFKL suppresses the phagocytic oxidative burst. Cell, Volume 184, Issue 17, 4480 - 4494.e15.
[4] Zhang, Y., Cao, Y., Wu, X. et al. Thermal proteome profiling reveals fructose-1,6-bisphosphate as a phosphate donor to activate phosphoglycerate mutase 1. Nat Commun 15, 8936 (2024). https://doi.org/10.1038/s41467-024-53238-w.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







