Breaking the Cataract Treatment Bottleneck: RNF114 Elucidates a New Mechanism of Reversible Lens Opacity Induced by Hypothermia
Cataract is a prevalent ophthalmic disorder caused by abnormal aggregation of lens proteins, leading to lens opacification, and remains one of the leading causes of blindness worldwide. The rapid advancement of the digital age has underscored the importance of eye health. Current treatment primarily involves surgical removal of the opaque lens, but the high costs and risks associated with surgery emphasize the need for non-surgical therapeutic approaches. Recently, a significant study was published in The Journal of Clinical Investigation, which discovered that the lens of thirteen-lined ground squirrel (GS; Ictidomys tridecemlineatus) becomes opaque at 4°C but rapidly restores its transparency upon re-warming to 37°C. This reversible phenomenon is regulated by a key molecule, RNF114, revealing a potential molecular target for therapeutic intervention. RNF114 (RING Finger Protein 114), an E3 ubiquitin ligase containing a RING finger domain, plays a pivotal role in protein degradation and cellular signaling. It regulates protein stability, activity, and subcellular localization by conjugating ubiquitin molecules to specific target proteins, thereby influencing various cellular processes. This research marks the first time RNF114 has been shown to regulate temperature-dependent changes in lens proteins, offering a potential target for the development of non-invasive cataract treatments. This finding not only enhances our understanding of cataract pathogenesis but also holds promise for the development of novel therapies, expanding our knowledge of the disease's mechanisms and providing new hope for non-surgical interventions.

Figure 1.
Sample Selection
GS-derived induced pluripotent stem cells (GS iPSCs) were utilized to generate GS-derived induced lens epithelial cells (iLEC); human lens epithelial cells (HLECs); GS and rat lens capsule tissues containing lens epithelial cells underwent proteomic analysis.
Technical Methods
1. qPCR
To assess lens protein expression in the iLEC model.
2. TMT Quantitative Proteomics
To identify differential lens proteins in GS and rat low-temperature models.
3. Pull-down-MS/CoIP-WB
To examine specific gene expression.
4. Immunofluorescence Imaging
5. Cell Viability Assay
Research Results
1. Reversal of GS Lens Opacity During Low-Temperature Rewarming Cycles
The study observed that during hibernation or torpor, GS lenses become opaque, but upon awakening, the opacity rapidly resolves (Figure 2A). To confirm this unique adaptation, the study compared the transparency of GS and rat lenses subjected to low-temperature rewarming. Under normal conditions, rat lenses are transparent and colorless, whereas GS lenses are transparent but pale yellow. Both GS and rat lenses exhibited significant opacity at 4°C for 24 hours, with central nuclear opacification being most prominent. After rewarming at 37°C for about 5 minutes, GS lenses returned to their pre-treatment transparency. In contrast, rat lenses retained notable nuclear opacity, with transmittance recovery limited to 86%. Extended rewarming did not further improve clarity (Figure 2B-D).
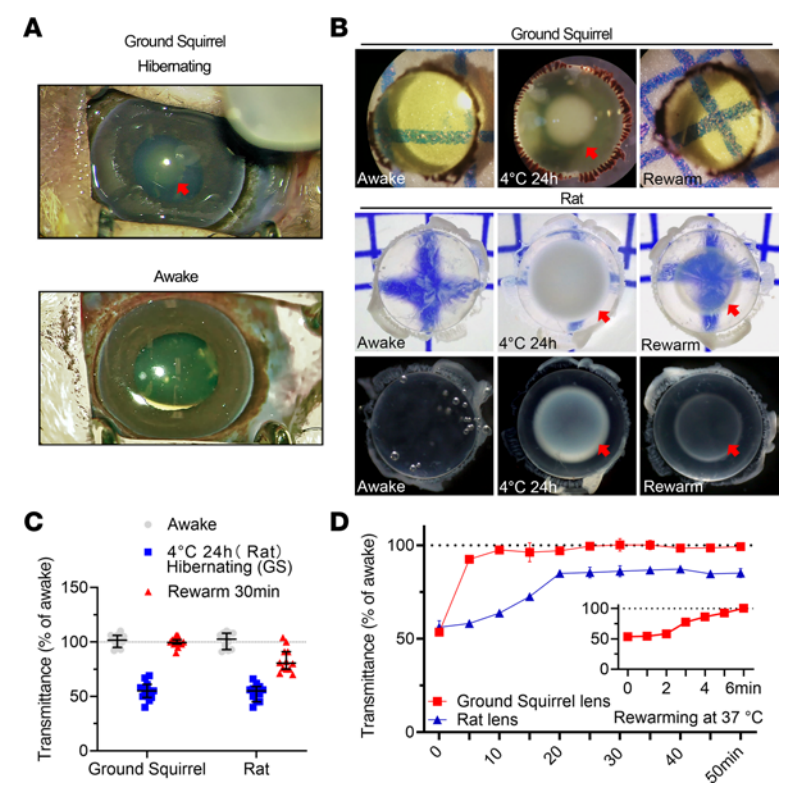
Figure 2. Transparency Results of GS and Rat Lenses After Low-Temperature Treatment and Rewarming
2. Proteomics Reveals That UPS Promotes the Degradation of Aggregated Lens Proteins
GS-derived iPSCs were used to establish iLEC models in vitro, and successful modeling was indicated by the increased expression of lens proteins (e.g., CRYAA, CRYAB, CRYBB2).
To explore the cold adaptation mechanism of hibernating animal lenses, TMT proteomics was employed to analyze lens capsule tissues containing lens epithelial cells from GS and rat models. Results showed that protein ubiquitination, ubiquitin-protein ligase activity, and proteasomal ubiquitin-dependent protein degradation were significantly enriched in GS after rewarming (Figure 3A). Various E1, E2, and E3 enzymes involved in the ubiquitin-proteasome system (UPS) were upregulated (Figure 3B). These findings suggest that the reversal of GS lens opacity during low-temperature rewarming may be linked to protein degradation mediated by the UPS. Prior studies have indicated that proteins are degraded through the UPS or autophagy in mammalian cells. The use of inhibitors for both pathways showed that the reduction in mutant CRYAA (Y118D) levels after rewarming was significantly inhibited by the proteasome inhibitor MG132. Live-cell imaging further revealed that MG132 inhibited the clearance of fluorescent spots from mutant αA-crystallin and αB-crystallin. These observations highlight the crucial role of the proteasomal UPS in the clearance of mutant αA-crystallin, while autophagy via lysosomes had no impact (Figure 3 I-L).
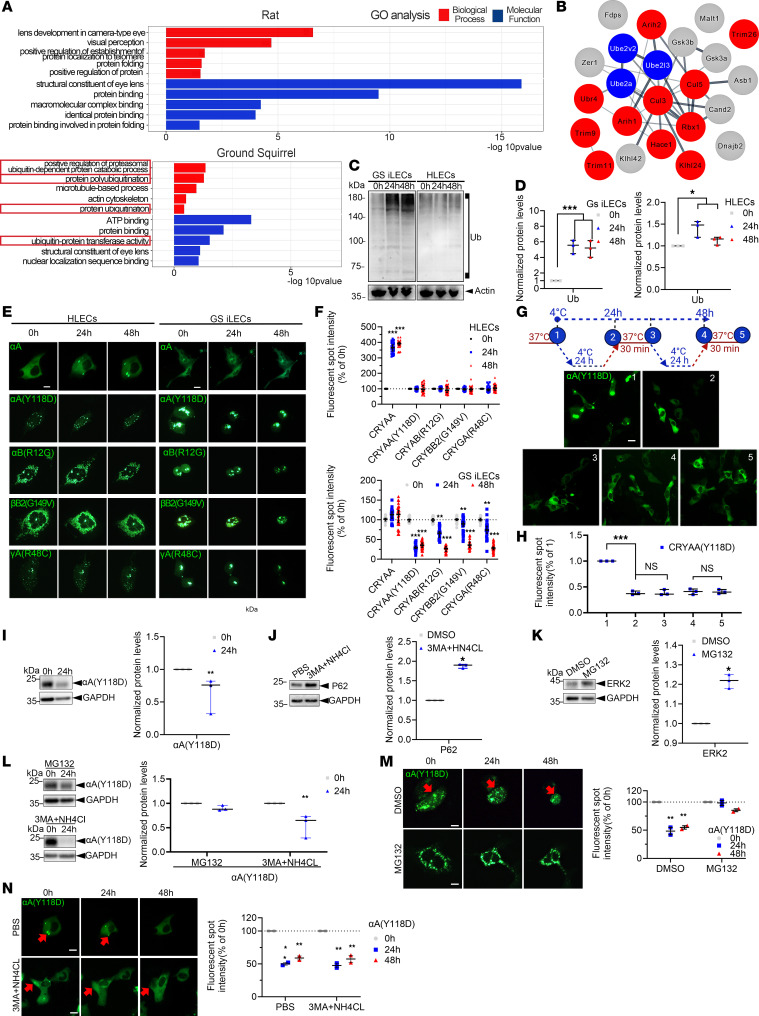
Figure 3. Protein Complexes May Alter Their Assembly State Under DNA Replication Stress
3. RNF114 Promotes CRYAA Ubiquitination During Low-Temperature Rewarming in GS iLECs
The study also investigated the stability of endogenous crystallins during the low-temperature rewarming cycle, finding that only CRYAA formed significant aggregates, while other crystallins (CRYAB, CRYBB1, CRYBB2, CRYBB3, γ-crystallins) did not exhibit noticeable aggregation (Figure 4A). For endogenous CRYAA, the study confirmed its interaction with ubiquitin through immunofluorescence and Co-IP (Figure 4B-D). After rewarming, Co-IP analysis revealed a marked increase in polyubiquitinated CRYAA levels in GS iLECs. Immunofluorescence further confirmed the co-localization of ubiquitin and CRYAA fluorescent spots. To identify potential E3 ubiquitin ligases interacting with CRYAA during rewarming, pull-down and mass spectrometry experiments revealed over 100 proteins specifically binding CRYAA in the rewarming group, one of which, RNF114, is known to possess E3 ligase activity. To further investigate RNF114's role in lens opacity reversal, mRNA levels of RNF114 and its interacting proteins were compared before and after low-temperature rewarming, revealing that RNF114 mRNA levels were elevated in GS iLECs, suggesting a role for RNF114 in adaptive regulation of GS lens clarity (Figure 4E). Co-IP confirmed the interaction between RNF114 and CRYAA after rewarming in GS iLECs (Figure 4F-H). Downregulation of RNF114 resulted in reduced polyubiquitination of CRYAA, while overexpression of RNF114 WT significantly promoted the reduction of CRYAA (Y118D) levels. The study also generated RNF114ΔC(1-200), which lacks the ubiquitin-binding motif (Figure 4I), and found that the introduction of RNF114ΔC did not increase CRYAA levels (Figure 4I-L). These findings confirm that RNF114 promotes CRYAA proteasomal degradation in HLECs.
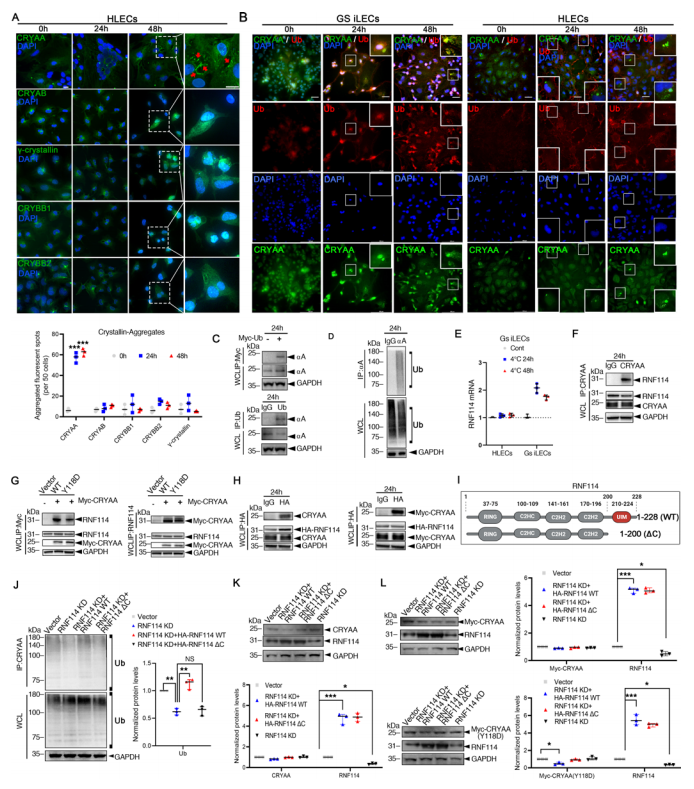
Figure 4. RNF114 Promotes CRYAA Ubiquitination During Low-Temperature Rewarming in GS iLECs
4. RNF114 Reverses Cataracts in Non-Hibernating Subjects
The study demonstrated that RNF114 did not affect the viability of HLECs or GS iLECs (Figure 5A and B), indicating the safety of RNF114 treatment for cold-induced cataracts. To enhance the delivery of RNF114 to HLECs and rat lenses, RNF114 was conjugated with a cell-penetrating peptide TAT-(47-58), derived from the HIV-1 TAT protein, which has been proven effective across various systems, including the eye. Immunofluorescence imaging confirmed that TAT-RNF114 entered the cytoplasm of HLECs and ex vivo rat lenses within 30 minutes of incubation (Figure 5C). When TAT or TAT-RNF114 was incubated with GFP-CRYAA (Y118D)-knockin HLECs for 4 hours, fluorescent aggregates in the cytoplasm gradually decreased, especially in CRYAA(Y118D) HLECs (Figure 5D). After incubating rat lenses at 4°C for 24 hours to induce cataract formation, TAT-RNF114 treatment rapidly reversed lens opacity, while control lenses remained opaque. Transmittance measurements also showed that TAT-RNF114 treatment significantly improved lens transparency during the low-temperature rewarming process (Figure 5E). Additionally, when cataracts were induced in zebrafish using H2O2 (oxidative stress), TAT-RNF114 treatment reduced lens opacity after 12 hours, whereas neither the TAT-RNF114ΔC group nor the TAT control group exhibited such effects. In the cataract grading system (LOCS III), 4 out of 5 zebrafish showed at least one level of improved transparency (Figure 5F), suggesting that RNF114 can alleviate cataract formation induced by stress beyond cold exposure.
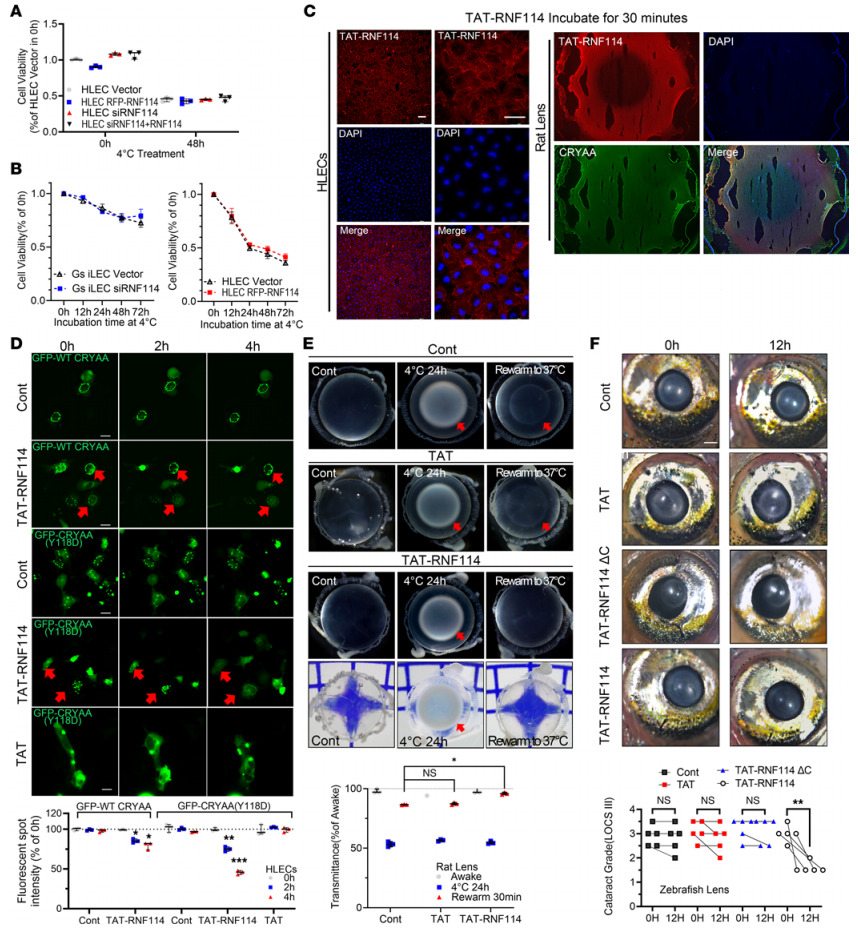
Figure 5. RNF114 Reverses Cataracts in Non-Hibernating Subjects and Demonstrates Safety for Cold-Induced Cataracts
Research Conclusion
Disruptions in protein homeostasis and abnormal protein aggregation, driven by various factors, are fundamental pathological changes underlying cataract formation. Through an investigation of the mechanism responsible for the reversible temperature-dependent changes in lens clarity in ground squirrels, this study reveals that these changes primarily rely on the upregulation of CRYAA aggregates during rewarming, mediated by the ubiquitin-proteasome system (UPS). The newly characterized RNF114 plays a central role in the degradation and turnover of these aggregates, showing significant reversibility in different stress-induced cataract models. These findings suggest that screening for and designing drugs that activate specific protein degradation pathways may offer a way to precisely regulate protein stability and turnover, potentially advancing the treatment of cataracts and other protein aggregation-related diseases.
MtoZ Biolabs is committed to providing comprehensive proteomic mass spectrometry services, including TMT quantitative proteomics, DIA quantitative proteomics, and targeted proteomics platforms, for researchers. We offer efficient and precise solutions to uncover the molecular mechanisms behind various diseases and foster innovation in the biomedical field. We welcome collaboration!
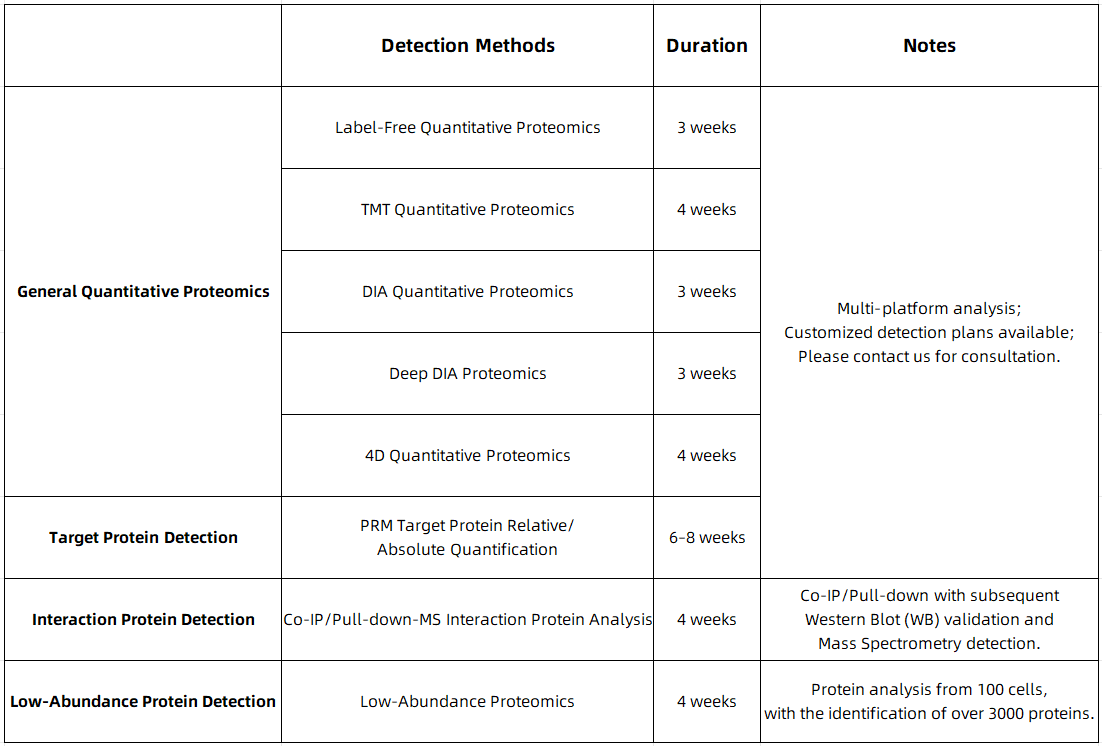
Figure 6.
References
[1] The Journal of Clinical Investigation ( IF 13.3 ) Pub Date : 2024 Sep 17 , DOI: 10.1172/jci169666
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







