Drug Target Identification and Validation Services
Drug target identification and validation are critical steps in the drug discovery process, forming the foundation for the development of effective therapeutics. This process involves discovering molecular targets that play key roles in disease mechanisms and confirming their relevance to the disease pathology. The identification of these targets provides a pathway for creating more specific and effective therapies. However, challenges such as complex disease pathways and off-target effects complicate this process, emphasizing the need for precise and reliable target validation.
Drug target validation ensures that identified targets are not only relevant but also modifiable by drugs, thus reducing the risk of clinical trial failures. Validating targets through techniques like genetic manipulation, protein-protein interaction studies, and small molecule screening is essential for confirming their functional significance in disease progression. This step is crucial in building a robust foundation for therapeutic development and minimizing the risks associated with drug resistance and side effects.
Service at MtoZ Biolabs
MtoZ Biolabs specializes in offering comprehensive Drug Target Identification and Validation Services designed to support pharmaceutical research and drug development. We provide highly accurate and specific data to identify and validate drug targets, uncovering the underlying mechanisms of action and therapeutic efficacy. Our Drug Target Identification and Validation Services utilize state-of-the-art techniques, enabling the precise detection of drug-target interactions and associated post-translational modifications. Our expertise in drug target identification and validation empowers pharmaceutical companies to accelerate their drug discovery pipelines and ensure the development of safe, targeted therapies.
MtoZ Biolab's drug target identification and validation scheme include:
1. Activity-Based Protein Profiling (ABPP)
Activity-Based Protein Profiling (ABPP) is a powerful method used for identifying drug targets by capturing and isolating proteins that bind to a specific drug or compound. This technique involves labeling a drug with a chemical tag that allows it to bind covalently to its target proteins. ABPP provides a means to identify target proteins directly within complex biological systems by isolating and analyzing drug-protein interactions. It is especially useful for mapping the interaction landscape of small molecules or biologics, facilitating the discovery of novel drug targets and mechanisms of action. ABPP helps validate drug-target engagement and assists in functionalizing therapeutic compounds.
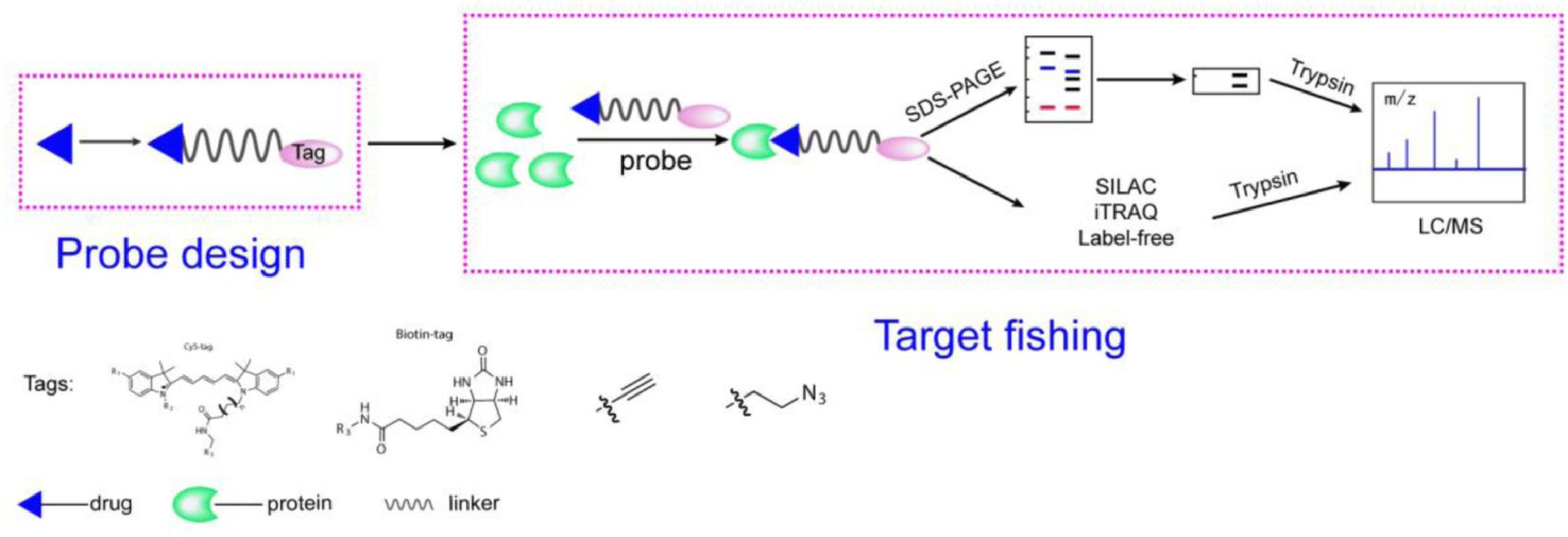
Figure 1. A General Representation of the ABPP Workflow
2. Thermal Proteome Profiling (TPP)
Thermal Proteome Profiling (TPP) is a technique that helps identify drug targets by measuring changes in protein stability induced by small molecule binding. TPP involves subjecting a protein sample to gradual heat, monitoring the stability of proteins, and identifying ligand-induced shifts in thermal stability. When a drug binds to its target protein, it typically alters the protein's thermal denaturation profile. This shift can be detected and used to identify drug-protein interactions and validate potential drug targets. TPP is valuable in target deconvolution, identifying off-target effects, and confirming drug-target specificity, making it a critical method in early-stage drug development.
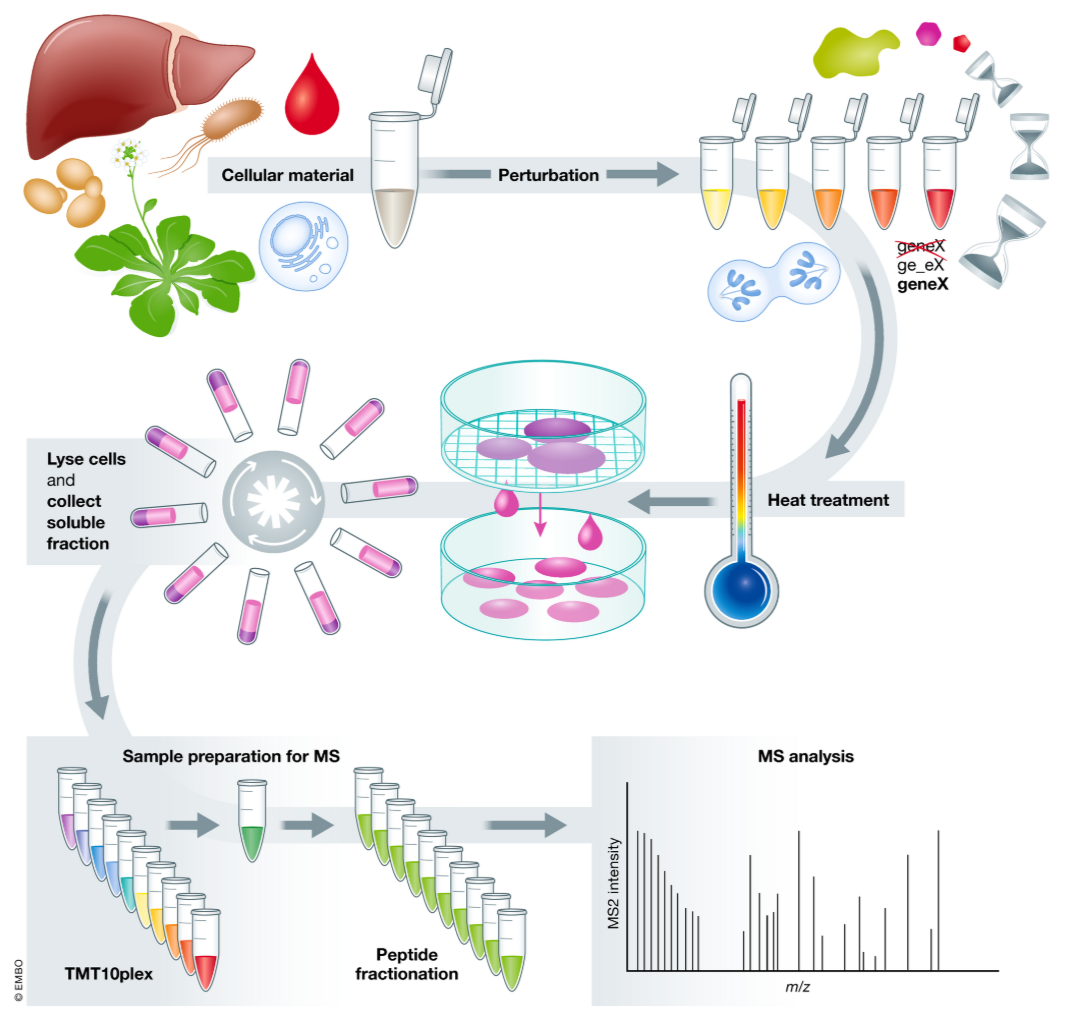
Mateus, A. et al. Mol Syst Biol. 2020.
Figure 2. Thermal Proteome Profiling (TPP) Experimental Setup
3. Limited Proteolysis-Mass Spectrometry (LiP-MS)
Limited Proteolysis-Mass Spectrometry (LiP-MS) is a technique used to assess protein conformation changes upon drug binding. In this method, proteins are partially digested with a protease, and the peptides formed are analyzed by mass spectrometry. Binding of a drug to a target protein can alter the protein’s structure, making some regions more or less accessible to the protease. This results in specific proteolytic cleavage patterns, which can be used to identify protein-ligand interactions and confirm drug binding sites. LiP-MS is particularly useful for identifying protein conformational changes, aiding in the discovery of functional drug targets and their binding sites.
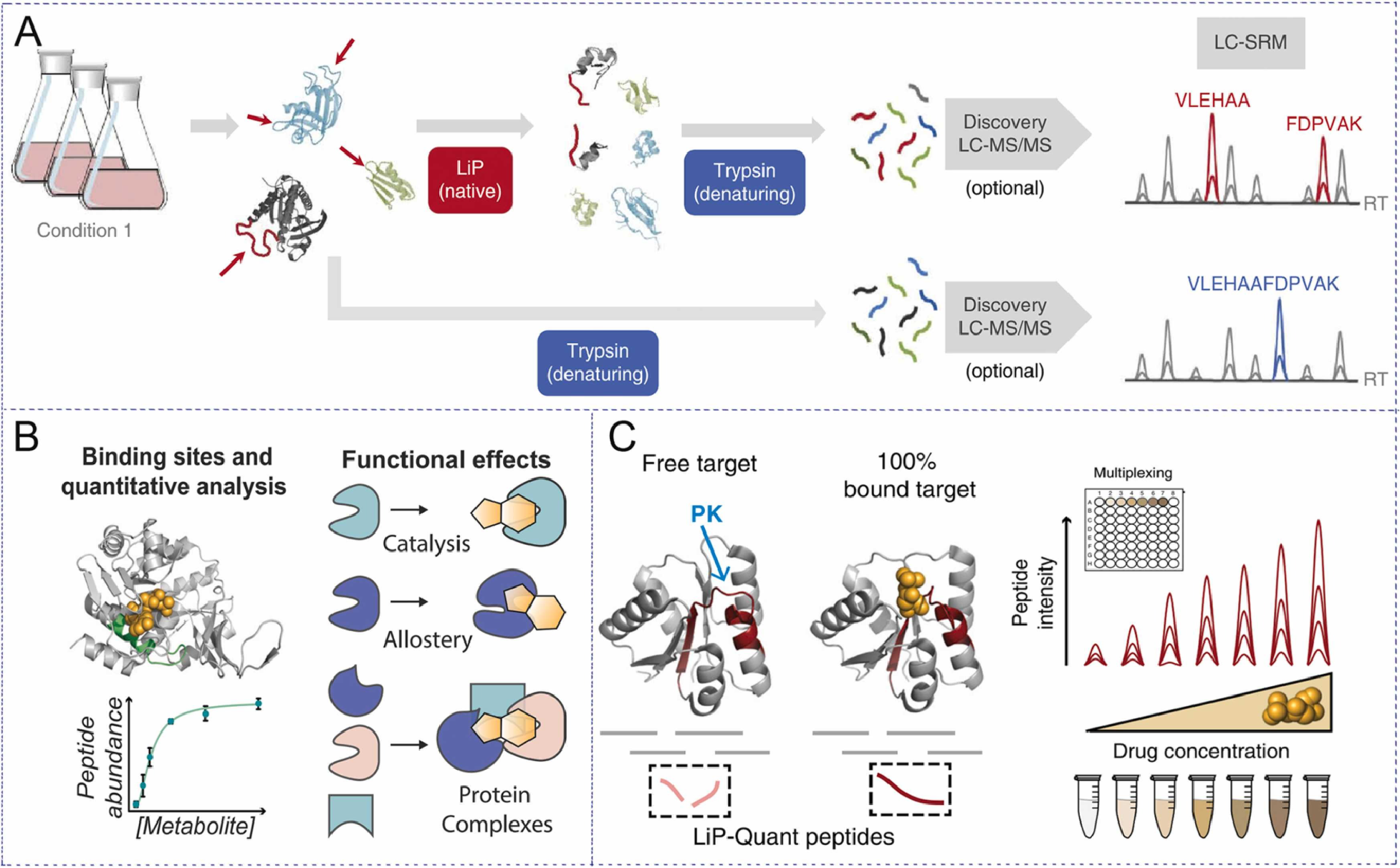
Feng, F. et al. J Pharm Biomed Anal. 2023.
Figure 3. Experimental Principles of LiP-MS
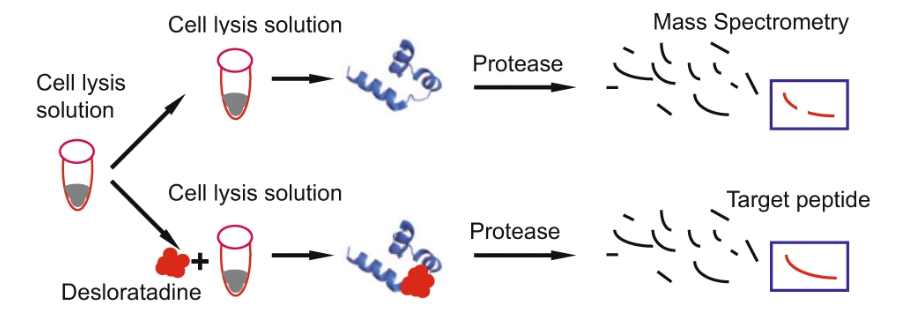
4. Drug Affinity Responsive Target Stability (DARTS)
Drug Affinity Responsive Target Stability (DARTS) is a method used to identify and validate drug targets by measuring ligand-induced changes in protein stability. In DARTS, proteins are incubated with small molecule drugs, and the stability of these proteins is monitored under conditions of limited proteolysis. If a protein binds to a drug, it becomes more resistant to proteolysis, making it possible to identify drug-bound proteins in complex samples. DARTS helps uncover novel drug targets, validate target engagement, and determine off-target effects, providing key information for drug target identification and validation. This technique plays a vital role in confirming the therapeutic relevance of drug-protein interactions.
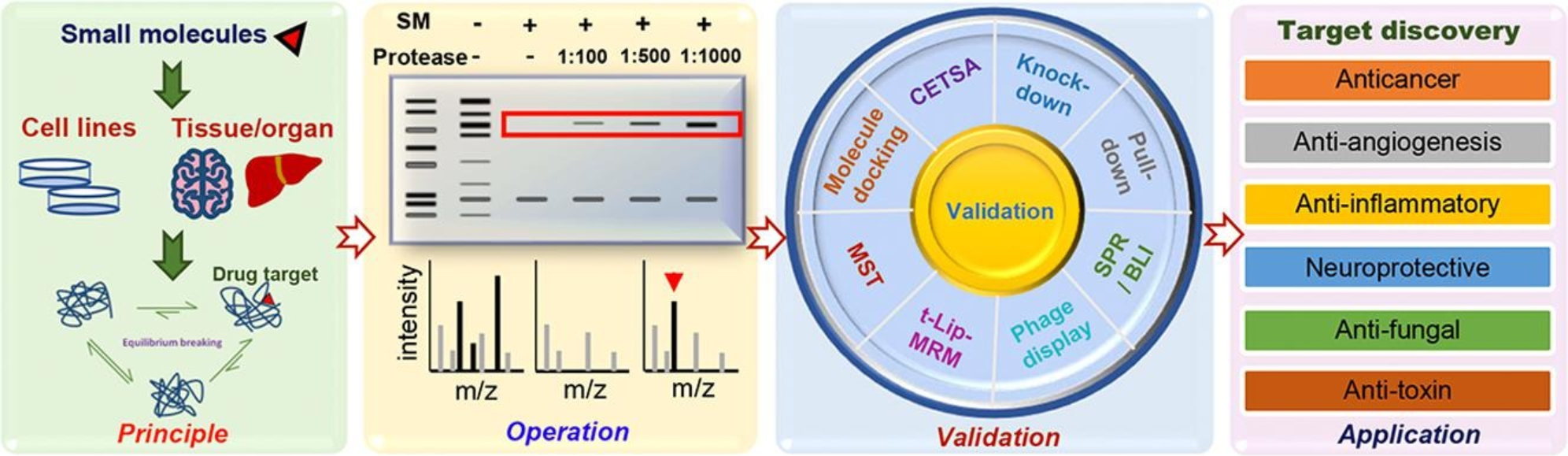
Ren, Y. S. et al. Biochem Pharmacol. 2021.
Figure 4. Principle, Experimental Process, and Applications of DARTS
Service Advantages
✔️High Sensitivity and Specificity: Through advanced mass spectrometry and affinity-based approaches, we achieve precise detection of drug-target interactions, ensuring that only relevant, disease-specific targets are identified.
✔️Customized Solutions: MtoZ Biolabs offers tailored Drug Target Identification and Validation Services to meet the specific needs of pharmaceutical and biotech companies, whether for small molecule drugs, biologics, or combination therapies.
✔️Cutting-edge Technology: Leveraging the most advanced proteomic and genomic technologies, our Drug Target Identification and Validation Services are at the forefront of drug discovery, offering high-precision data and actionable insights for developing next-generation therapeutics.
✔️Rapid and Efficient: Our streamlined workflows ensure that target identification and validation are performed with quick turnaround times, facilitating the acceleration of your drug discovery process.
Case Study
In a recent study, researchers successfully identified and validated a novel drug target for Hepatocellular Carcinoma (HCC) using a library of 419 FDA-approved compounds. Through high-throughput screening and mass spectrometry-based proteomics, desloratadine, an antiallergic drug, was identified as a potential anticancer agent. Drug Affinity Responsive Target Stability (DARTS) and Surface Plasmon Resonance (SPR) assays revealed N-myristoyl transferase 1 (NMT1) as its target protein. Functional assays showed that NMT1 promoted tumor growth, and its inhibition suppressed HCC progression in both in vitro and in vivo models. Additionally, NMT1-mediated myristoylation of Visinin-like protein 3 (VILIP3) was shown to regulate NFκB/Bcl-2 signaling, contributing to tumor survival. This study highlights NMT1 as a potential therapeutic target and biomarker for HCC, showcasing the importance of drug target identification and validation in cancer therapy.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Relevant Liquid Chromatography and Mass Spectrometry Parameters
4. The Detailed Information of Drug Target Identification and Validation
5. Mass Spectrometry Image
6. Raw Data
Contact us to explore tailored solutions for your research.
How to order?







