Gene Knockout for Drug Screening and Target Identification
A key step in drug discovery is target identification—identifying "druggable" biological targets. After a target is identified, it must be validated to demonstrate the functional relationship between the target and disease phenotype while ensuring safety. The drug discovery process is lengthy and costly, but good target validation increases the likelihood of developing effective drugs and achieving clinical success. The latest developments in CRISPR-Cas9 gene editing technology have had a significant impact on drug discovery, allowing researchers to intentionally activate or inhibit genes to elucidate and understand cellular pathways that play roles in disease progression. It is also used to create accurate models for better studying disease phenotypes and screening small molecules to quickly identify numerous potential targets.
Once these potential targets are identified, mass spectrometry can be used to study the interactions between potential drug molecules and their target proteins or metabolic products, revealing how drugs bind to their targets and how this binding affects intracellular signaling and metabolic pathways. By combining high-throughput gene knockout technology and mass spectrometry analysis, researchers can quickly identify new drug targets and gain a deeper understanding of the mechanisms of drugs, thus designing more effective and safer treatment methods.
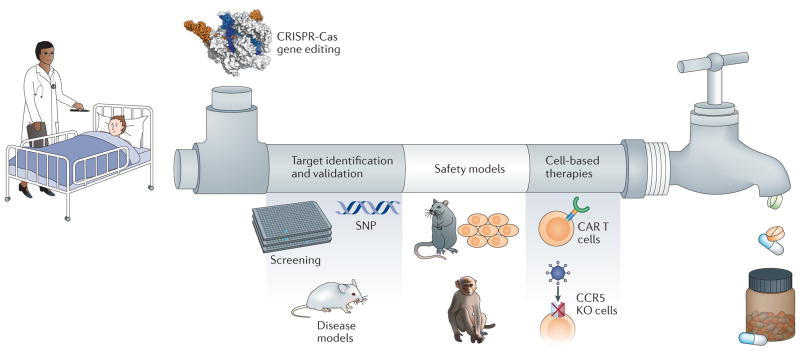
Fellmann, C. et. al. Nat. Rev. Drug. Discov. 2017.
Figure 2. Application of High-Throughput Gene Knockout in Drug Discovery
MtoZ Biolabs, based on high-throughput gene knockout technology combined with high-throughput mass spectrometry platform, can provide you with a one-stop solution from gene knockout to drug target identification. Our optimized CRISPR/Cas9 system can achieve single/multiple gene knockout, frameshift mutation, and large sequence deletion in human/mouse cell lines, primary cells, immune cells, and iPS cells. MtoZ Biolabs aims to provide you with the highest quality scientific research services, please feel free to contact us for more details of our services!
Service Advantages
1. Knockout Efficiency Guarantee
Optimized CRISPR/Cas9 system, over 80% target knockout efficiency at 70%; after gene knockout, we use Sanger sequencing and deep proteomics technology to double-verify the knockout efficiency, double guaranteeing the authenticity of the data.
2. Various Target-Based Drug Target Identification Platforms
We rely on high-resolution mass spectrometry platform, combined with affinity chromatography technology and active site-directed probes, to develop and validate various target-based drug target identification platforms, meeting different scientific research needs.
3. Short Project Cycle
Before conducting gene knockout experiments, we analyze the necessity and expression of genes in different cells to ensure the feasibility of the experiment and reduce the risk of experimental failure; and there is no need for gRNA validation for customers' targets in pre-experiments, greatly shortening the project cycle.
4. One-Stop Service
MtoZ Biolabs has rich experience in omics services and professional technical personnel, who can customize the optimal project plan according to your needs. You only need to tell us your experimental purpose and send samples, MtoZ Biolabs is responsible for all subsequent projects, including sample processing, experimental analysis, data analysis, and project reporting.
Applications
1. Whole-Genome CRISPR-cas9 Knockout Screening Combined with Mass Spectrometry to Determine KRAS Mutant Colon Cancer MEK Inhibitor Resistance Targets
Targeting the KRAS pathway is a promising approach to treating colorectal cancer (CRC), but CRC cells can tolerate MEK inhibitors, making the clinical trial results of MEK inhibitors in CRC patients unsatisfactory. Therefore, the authors conducted whole-genome CRISPR/Cas9 screening in the presence of MEK inhibitors to identify genes that are synthetically lethal through MEK inhibition in a CRC model with KRAS mutations. The results showed that GRB7 mediates primary resistance to MEK inhibitors through the RTK pathway. Mass spectrometry analysis of GRB7 immunoprecipitates showed that PLK1 was the predominant interacting kinase of GRB7.. The combination of PLK1 and MEK inhibitors synergistically inhibited CRC cell proliferation and induced apoptosis in vitro and in vivo. Ultimately, the authors identified GRB7-PLK1 as the pivot mediating RTK, leading to MEK inhibitor tolerance. This study suggests that PLK1 is a promising target for synergizing MEK inhibitors in clinical treatment of CRC patients harboring KRAS mutations.
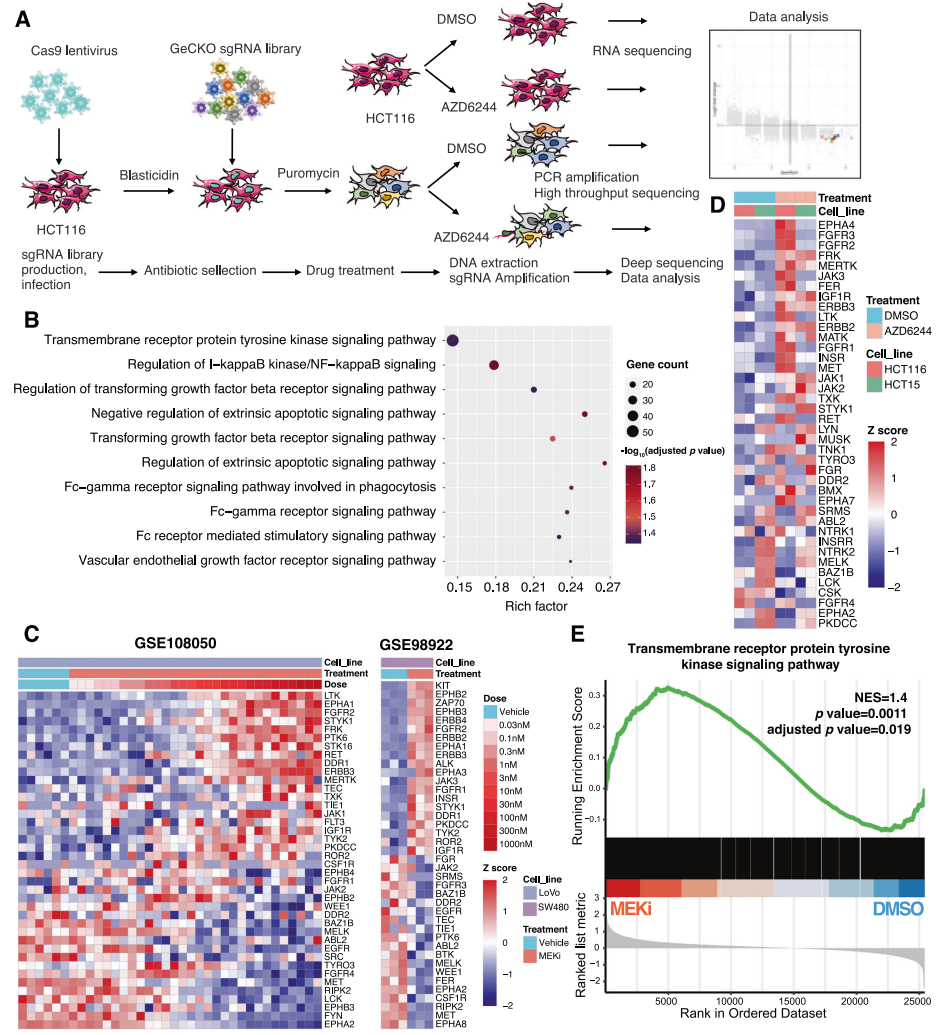
Yu, C., et al. Oncogene. 2022.
Figure 2. CRISPR Library Screening, Identifying RTK Pathway Involvement in KRAS Mutant Colon Cancer Cells' Resistance to MEK Inhibitors
2. Using CRISPR High-Throughput Screening for Next-Generation Candidate Drugs
Gefitinib and erlotinib are effective drugs for treating non-small cell lung cancer, targeting the epidermal growth factor receptor (EGFR). However, the acquired mutation EGFR C797S prevents the drug from interacting with the tyrosine kinase domain of EGFR, resulting in resistance to these drugs in patients. Astra Zeneca used CRISPR-Cas9 high-throughput gene knockout technology to create an accurate C797S mutation in cancer cell lines, and the C797S cell line model application enabled drug screening to identify compounds targeting this new mutation.
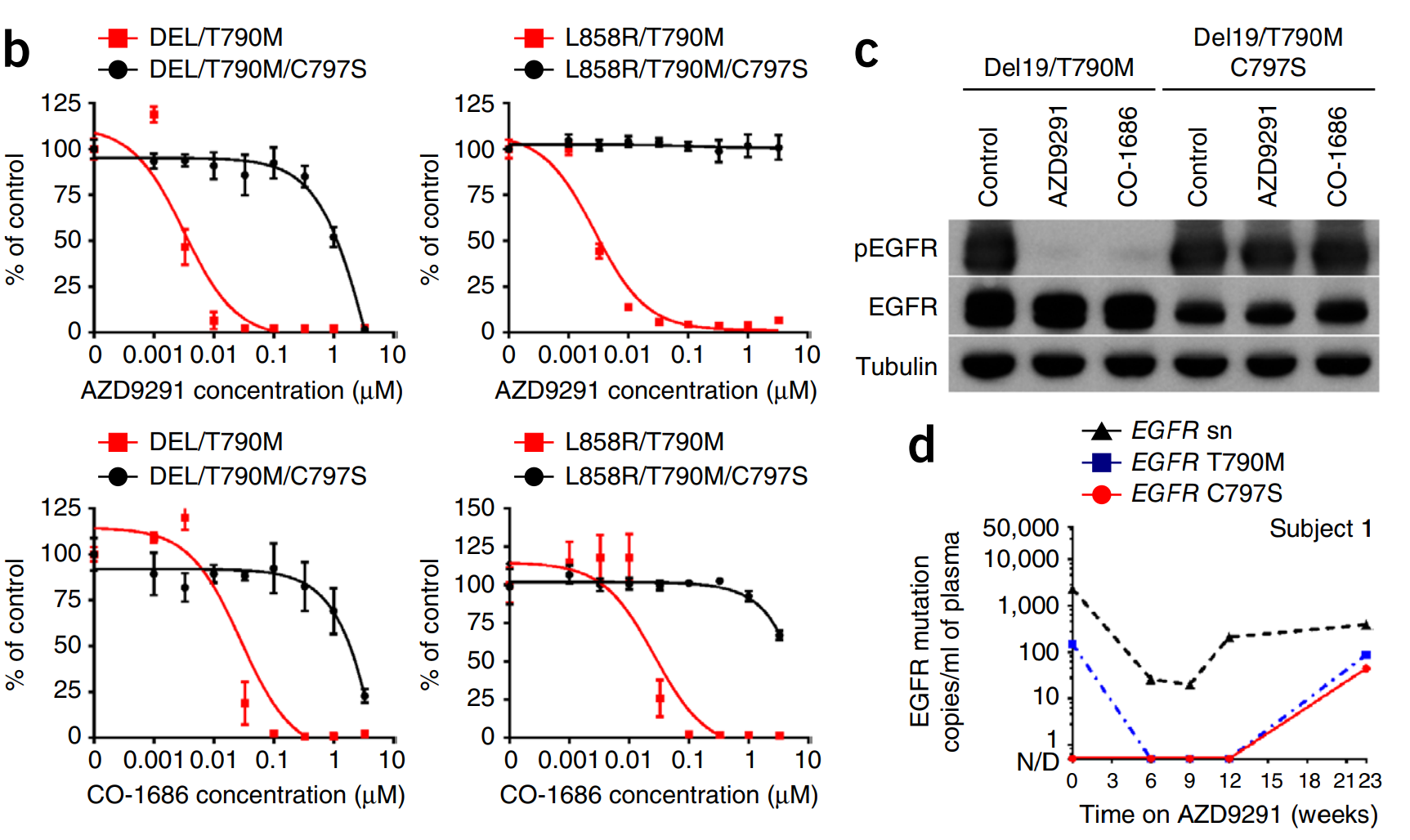
Thress, K. et al. Nat Med. 2015.
Figure 3. Acquired EGFR C797S Mutation Mediated Resistance to AZD929
How to order?







