HCP Quantification Service
- For high sensitivity and complex sample analysis, LC-MS is recommended.
- For high-throughput applications with moderate sensitivity, ELISA is ideal.
- For comprehensive profiling, 2D-DIGE offers superior resolution and dual qualitative-quantitative insights.
In the field of biopharmaceuticals and biotechnology, HCP (host cell protein) quantification is crucial for ensuring the purity, safety, and regulatory compliance of biological products. HCPs are non-target proteins produced by host cells during the manufacturing of recombinant proteins, monoclonal antibodies, or other biologics. Residual HCPs can compromise the quality of the final product and may pose risks to patient health. Comprehensive and rigorous HCP quantification enables the detection of these residual proteins, ensuring their levels remain within regulatory safety limits, and thereby safeguarding the quality and compliance of biopharmaceuticals.
Services at MtoZ Biolabs
MtoZ Biolabs offers full spectrum of HCP Quantification Service designed to support efficient and precise measurement of residual HCPs in biopharmaceuticals. Employing state-of-the-art techniques, we provide both relative and absolute quantification to meet diverse experimental needs and quality standards. Our commonly used methods include:
1. 2D-DIGE
This high-resolution technique combines advanced protein separation with differential labeling, enabling precise identification and quantification of HCP residues. It is particularly suitable for HCP quantification in complex samples, offering dual qualitative and quantitative insights.
2. ELISA
ELISA is a widely adopted technique known for its high sensitivity and efficiency in detecting specific HCP concentrations. By leveraging anti-HCP antibodies, it provides reliable relative quantification and is ideal for high-throughput sample screening.
3. LC-MS
LC-MS integrates the separation capabilities of liquid chromatography with the high sensitivity and specificity of mass spectrometry. This allows for precise absolute quantification of HCPs, making it ideal for complex biological samples and low-abundance proteins.
4. Western Blotting
As a classic protein detection method, Western Blotting validates the presence of HCPs while estimating their relative abundance through standardized band analysis. It is a versatile tool for both qualitative and semi-quantitative assessment across various biologics.
Analysis Workflow
To ensure data accuracy and reproducibility, our HCP Quantification Service follow a rigorous workflow:
1. Sample Preparation: Extract HCPs from client-provided samples.
2. Quantitative Analysis: Apply advanced methods such as 2D-DIGE, ELISA, or LC-MS for precise HCP quantification.
3. Data Analysis: Process and interpret experimental data using specialized software, ensuring high accuracy and reproducibility.
4. Report Generation: Deliver detailed quantitative analysis reports with comprehensive result interpretation and actionable recommendations.
5. Result Validation and Consultation: Provide further validation if required, along with technical guidance to optimize manufacturing processes and reduce HCP residues.
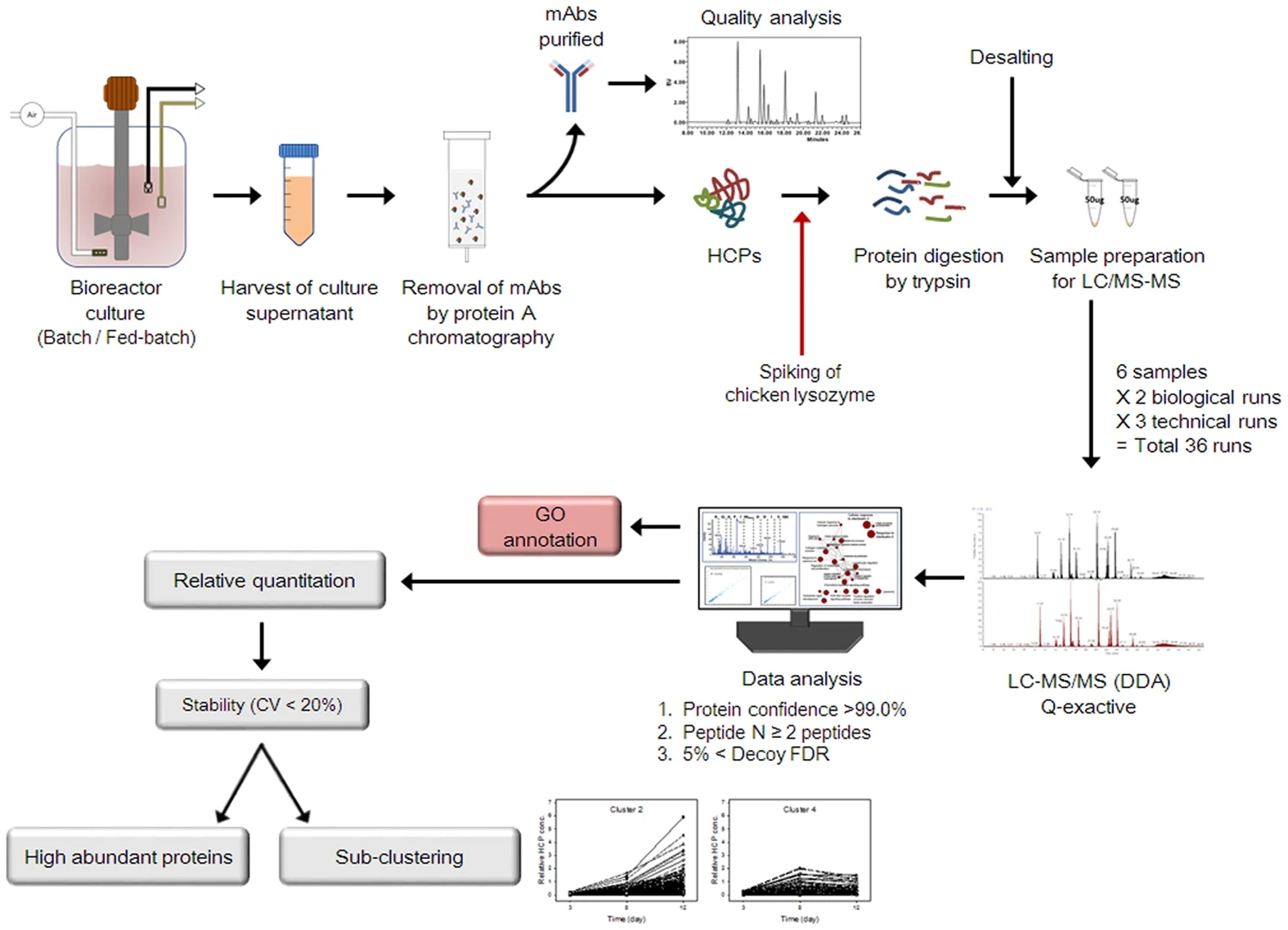
Park, J. H. et al. Sci. Rep. 2017.
Figure 1. HCP Quantification Workflow
Why Choose MtoZ Biolabs?
1. Advanced Analysis Platform
MtoZ Biolabs established state-of-the-art platforms to ensure accuracy and reliability in HCP Quantification Service.
2. Multi-Sample Compatibility
Our methods are compatible with a variety of host cells, including E. coli, CHO cells, and insect cells, ensuring broad applicability.
3. High-Data-Quality
Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrate all HCP quantification analysis data, providing clients with a comprehensive data report.
4. Customized Service
Tailored to the specific research needs of our clients, we offer flexible experimental design and personalized data analysis to ensure the achievement of research goals to the fullest extent.
5. One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
Our HCP Quantification Service is extensively utilized in the biopharmaceutical industry, including applications in recombinant protein drugs, monoclonal antibodies, vaccines, and other biologics. By providing precise and reliable HCP quantification, we assist clients in meeting global regulatory standards for drug safety and purity.
MtoZ Biolabs is dedicated to supporting clients in achieving the highest standards of quality during development, production, and regulatory review of biologics. Contact us today to explore how we can assist with your HCP quantification needs.
Case Study
Case 1: Precision Quantification of Host Cell Proteins Using DIA-MS
This study employed Data-Independent Acquisition Mass Spectrometry (DIA-MS) alongside an innovative HCP Profiler standard to establish a highly sensitive and accurate workflow for HCP quantification. The method achieved comprehensive coverage and precise detection of HCPs during monoclonal antibody (mAb) production, with sensitivity reaching sub-ng/mg levels and a coefficient of variation below 10%. The results demonstrated the effectiveness of this workflow in supporting biopharmaceutical process development and quality control, underscoring the value of HCP Quantification Service in ensuring drug safety and consistency.
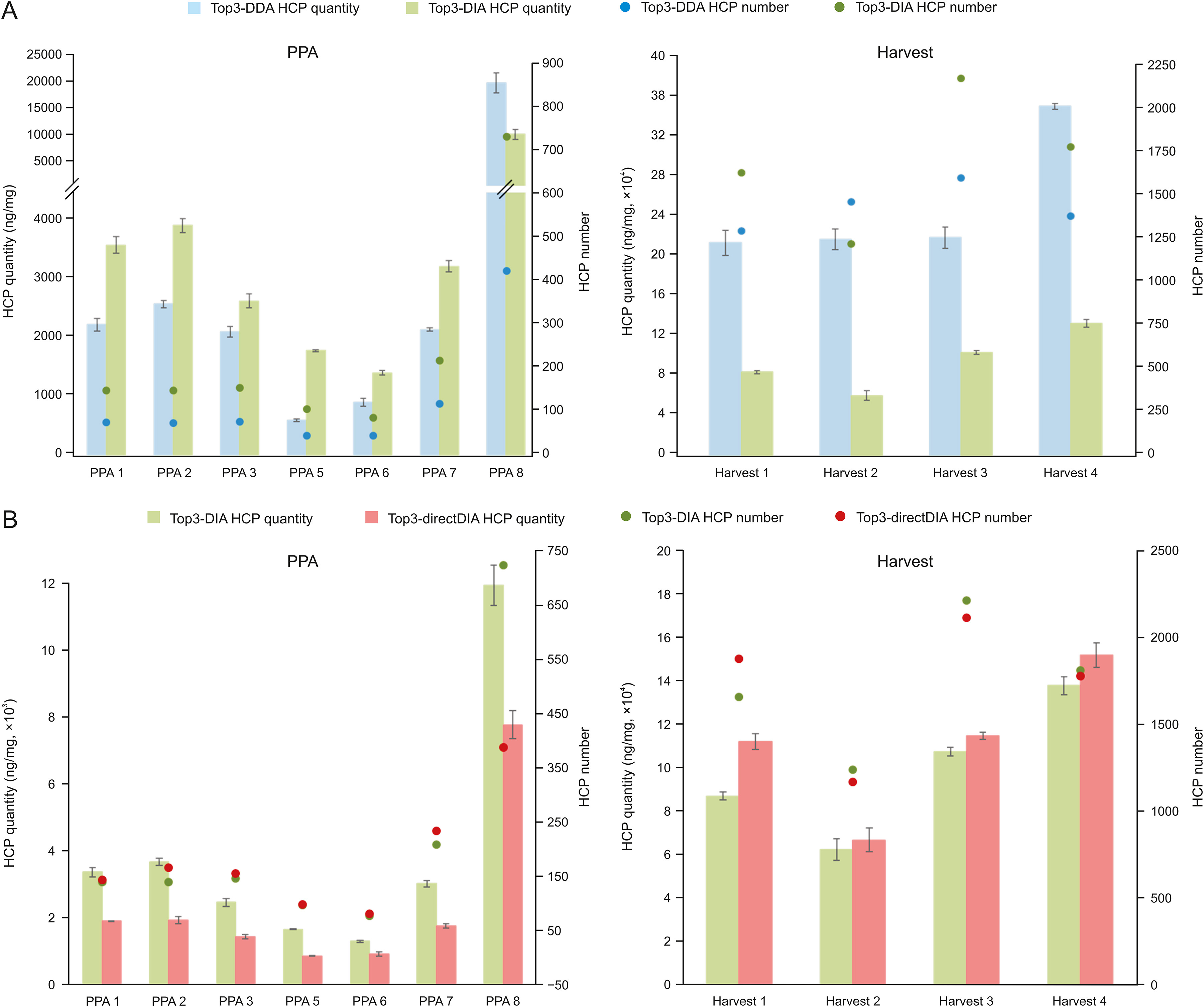
Hessmann, S. et al. J Pharm Anal. 2023.
FAQ
Q1: What distinguishes absolute quantification from relative quantification in HCP analysis?
Absolute HCP quantification determines the exact concentration of target proteins using a standard curve, yielding precise quantitative data. In contrast, relative HCP quantification compares sample HCP levels to a standard, producing data suitable for cases where precise absolute concentrations are not required. MtoZ Biolabs offers comprehensive HCP Quantification Service including both absolute and relative quantification methods, tailored to meet the diverse needs of biopharmaceutical research and quality control.
Q2: How should I choose the most appropriate HCP quantification method?
Selecting the right method depends on your specific requirements:
Our team of experts is available to provide personalized recommendations tailored to your project needs.
How to order?







