Host Cell DNA Analysis Service
- Biopharmaceutical Development: Ensures that recombinant proteins, monoclonal antibodies, and other biologics meet regulatory standards for residual host cell DNA.
- Quality Control: Enables real-time monitoring of host cell DNA levels throughout manufacturing processes, ensuring product purity and safety.
- Clinical Trial Preparation: Provides standardized host cell DNA analysis data for clinical trial products, supporting drug safety and regulatory compliance.
Host cell DNA (HCD) refers to trace residual DNA fragments derived from host cells (e.g., CHO cells, E. coli, yeast) used in producing therapeutic proteins, vaccines, monoclonal antibodies, and other biologics. Despite rigorous purification steps during manufacturing, small amounts of host cell DNA may persist in the final product.
Residual host cell DNA poses potential risks, including immunogenicity, oncogenicity, and viral contamination. Therefore, accurately and sensitively quantifying host cell DNA is essential throughout the biopharmaceutical research and manufacturing processes. International regulatory agencies have established strict limits for host cell DNA residues, typically requiring levels below 100 pg or 10 ng per dose, depending on the cell line type, administration route, and dosing frequency.
Comprehensive host cell DNA analysis ensures drug safety, efficacy, and batch-to-batch consistency, while also satisfying global regulatory compliance requirements, facilitating the successful commercialization of biopharmaceutical products.
MtoZ Biolabs offers efficient and precise Host Cell DNA Analysis Service utilizing highly sensitive real-time quantitative PCR (qPCR) platform in combination with a robust quality control framework. Our end-to-end offerings include sample preparation, DNA extraction, quantitative analysis, and data interpretation, ensuring compliance and product safety across drug development, production validation, and quality control stages.
Technical Principles
Quantitative polymerase chain reaction (qPCR) is a widely used technique for accurately measuring residual host cell DNA. Unlike conventional PCR, qPCR enables real-time monitoring of DNA amplification during every reaction cycle. Through the integration of fluorescent dyes or probes, qPCR provides early detection of amplified DNA, facilitating precise quantification.
This technique relies on specific primers and probes designed to target host cell-specific gene sequences (e.g., conserved regions within the host cell genome). During amplification, fluorescent signal intensity directly correlates with the initial quantity of DNA, allowing reliable quantitative analysis of residual host cell DNA.
Analysis Workflow
At MtoZ Biolabs, our Host Cell DNA Analysis Service ensures the detection and quantification of host cell DNA contaminants, a critical step in safeguarding product quality and regulatory compliance. Below is an overview of our comprehensive analysis workflow.
1. Sample Collection and Preparation
Samples, including cell culture supernatants, final products, and raw intermediates, are collected and processed using appropriate reagents to ensure efficient DNA extraction while preventing degradation.
2. DNA Extraction
Samples undergo physical or chemical lysis, and high-purity extraction kits are used to remove impurities, ensuring DNA quality and purity.
3. Primer and Probe Design
Specific primers and probes targeting host cell DNA sequences are designed to guarantee accurate and sensitive detection.
4. qPCR Reaction
Extracted DNA is combined with primers, probes, and fluorescent dyes, and DNA amplification is monitored in real time during each qPCR cycle.
5. Data Analysis and Reporting
Fluorescence signals are analyzed to quantify residual host cell DNA, and results are presented in detailed analysis reports.
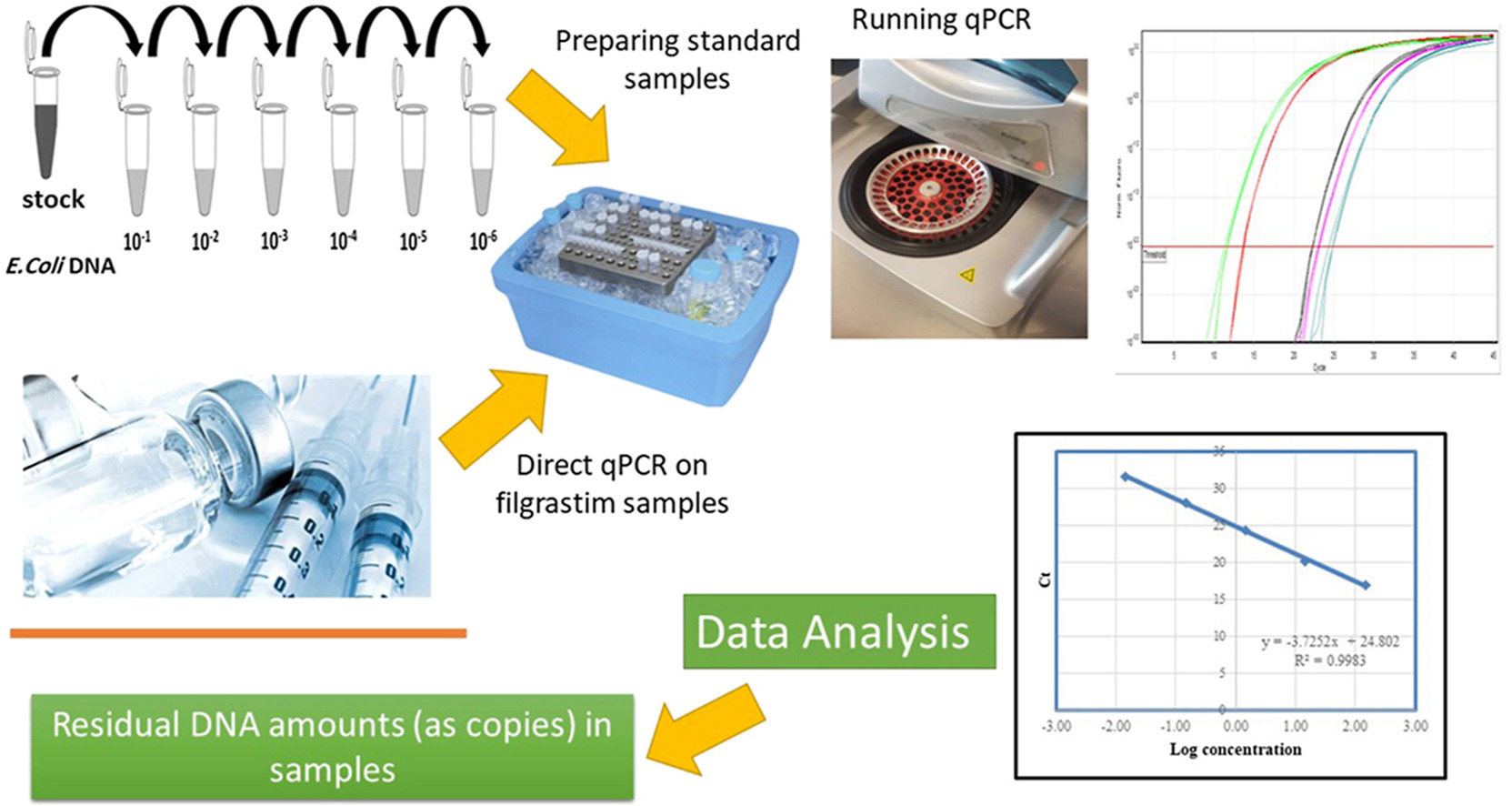
Gholizadeh-Hashjin, A. et al. Anal. Biochem. 2021.
Why Choose MtoZ Biolabs?
1. High Sensitivity and Accuracy: Capable of detecting pg-level genomic DNA, fg-level bacterial DNA, and virus-derived DNA.
2. Exceptional Specificity: Advanced primer and probe design ensure accurate differentiation between host cell DNA and other contaminants, minimizing cross-reactivity.
3. Robust Quantitative Capability: Provides precise concentration measurements of residual host cell DNA, meeting diverse quality control and regulatory compliance requirements.
4. Customized Service: Tailored to the specific research needs of our clients, we offer flexible experimental design and personalized data analysis to ensure the achievement of research goals to the fullest extent.
5. One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Sample Submission Suggestions
1. Sample Types
Cell culture supernatants, final products, raw intermediates, fermentation liquids, etc.
2. Sample Volume
Minimum 1 mL of liquid sample or an equivalent amount of solid sample (e.g., lyophilized powder)..
3. Sample Quality
Recommended DNA concentration of ≥10 ng/µL for optimal performance.
Note: For special requirements or sample preparation assistance, please contact us.
Applications
Our Host Cell DNA Analysis Service is vital for ensuring accurate detection of host cell DNA, supporting key applications such as:
Case Study
Case 1: Accurate Detection of Residual Host Cell DNA in Protamine Sulfate Drugs Using qPCR
This study developed a qPCR-based method for quantifying residual host cell DNA in protamine sulfate drug products. Through optimized qPCR parameters and the use of specific primers and probes, interference from complex drug matrices was mitigated. The results demonstrated stable and reliable detection of trace DNA levels, supporting quality control and safety evaluations, underscoring the critical role of Host Cell DNA Analysis Service in regulatory compliance.
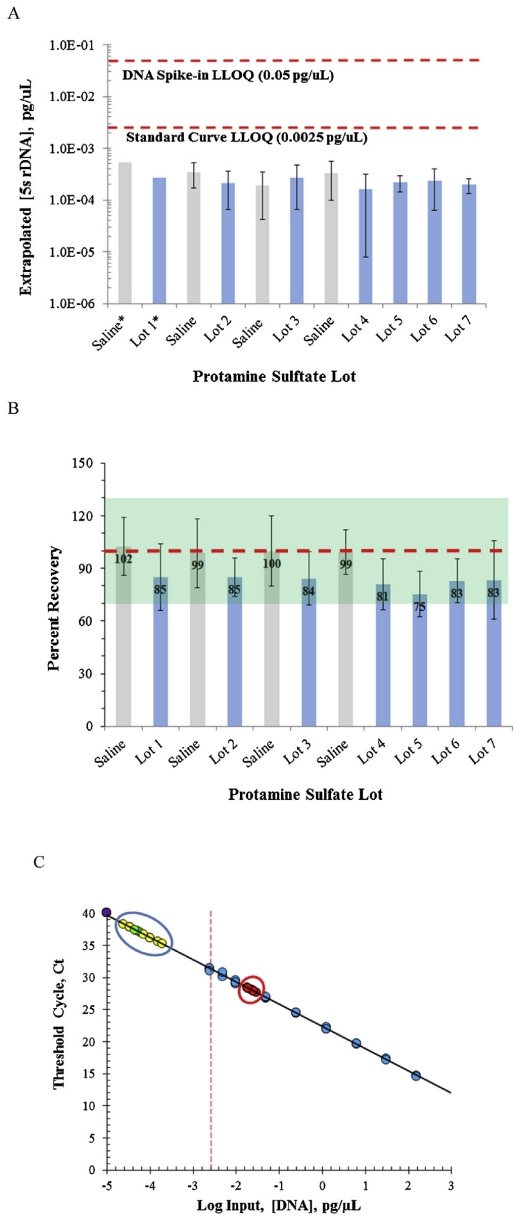
Sommers, C. et al. J. Pharm. Biomed. Anal. 2018.
Case 2: Precise Quantification of E. coli Residual Host Cell DNA Using qPCR
A qPCR method targeting the 23S ribosomal RNA gene was developed to detect E. coli residual host cell DNA efficiently. By employing specific primers and probes and optimizing the reaction conditions, the study achieved highly sensitive and specific detection of low-level residual DNA in complex samples. This method offers a robust quality control tool for biologics produced using E. coli expression systems.
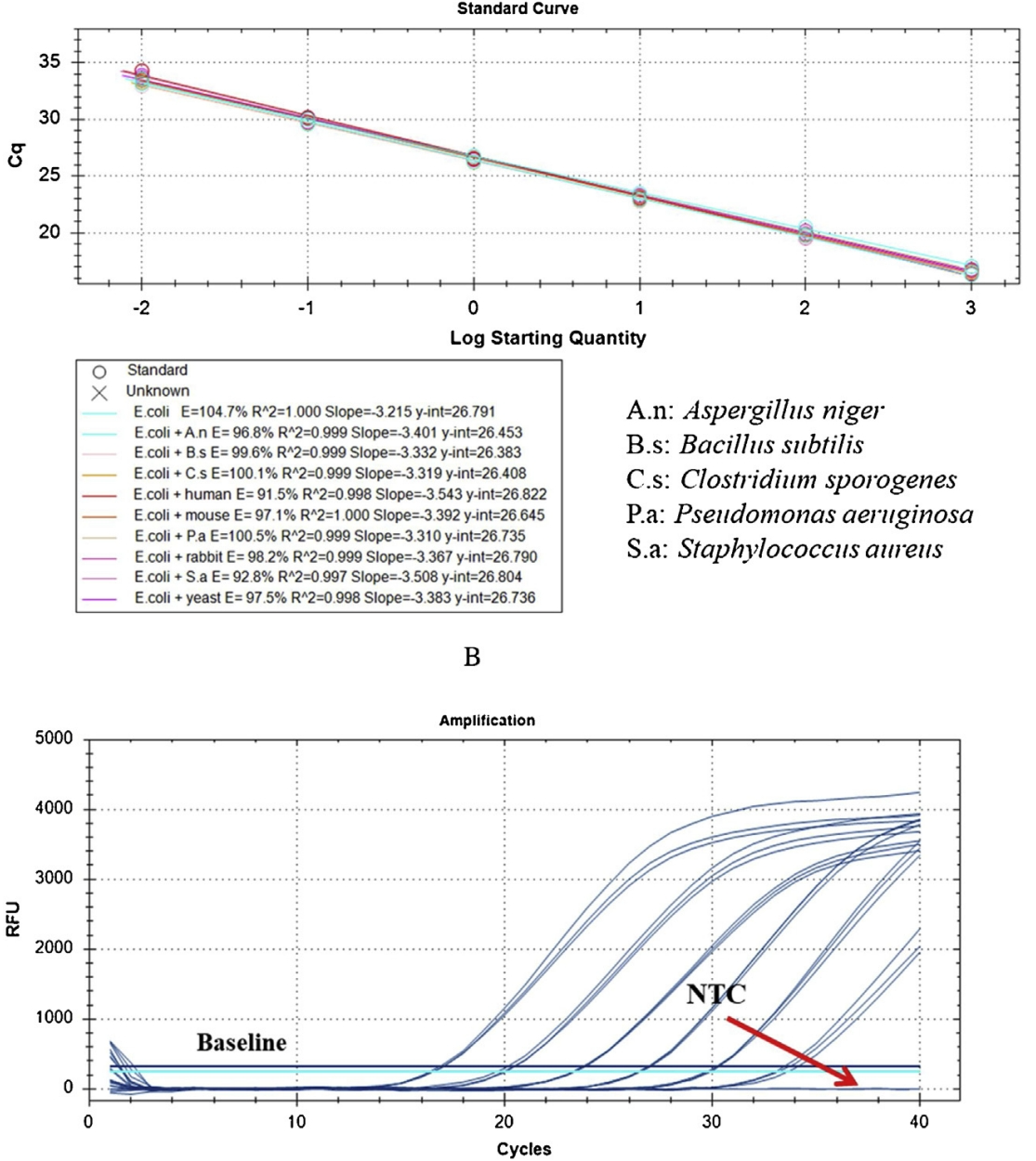
Li, D. et al. J. Pharm. Biomed. Anal. 2021.
FAQ
Q1: What types of host cell DNA can be detected by this service?
Our Host Cell DNA Analysis Service can detect residual DNA from diverse host cell systems, including:
· Mammalian cells (e.g., CHO, HEK293)
· Insect cell lines (e.g., Sf9, High Five)
· Bacterial cells (e.g., E. coli)
· Yeast cells
We offer customized primers and probes to ensure precise detection tailored to your production platform, meeting global pharmaceutical regulatory requirements.
MtoZ Biolabs’ Host Cell DNA Analysis Service powered by advanced qPCR technology and stringent quality control protocols, delivers highly accurate and efficient detection of host cell DNA. Our services support clients in ensuring regulatory compliance, product safety, and quality assurance across research, development, and manufacturing phases.
How to order?







