Host Cell Protein Assay Service
Host Cell Protein Assay Service is used to detect and quantify host cell-derived proteins present during the production of biopharmaceuticals. Host cell proteins (HCPs) are considered impurities in the manufacturing process, as they are not part of the intended product. This is particularly critical for biologics such as monoclonal antibodies, therapeutic enzymes, or vaccines, where HCPs can affect the quality, safety, and efficacy of the final product by triggering immune responses or interfering with drug activity. Therefore, Host cell protein assay is essential in biopharmaceutical production. Specifically, in biologics manufacturing, HCP detection is a crucial part of quality control and validation processes, ensuring the final product meets quality standards and complies with regulatory requirements.
Wohlrab S. et al. Am Pharm Rev. 2018.
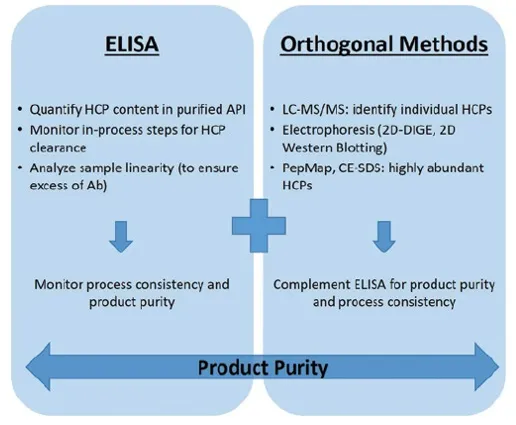
Host cell protein assay can be performed using various methods, including ELISA (Enzyme-Linked Immunosorbent Assay), Western Blotting, and mass spectrometry. Among these, ELISA is the most widely used and serves as the standard method for HCP detection. By employing antibodies specific to host cell proteins, ELISA measures signal intensity upon binding, enabling the detection of HCPs. ELISA is characterized by its high sensitivity and simplicity, and is suitable for large-scale sample screening. Leveraging high-precision UV-Vis spectrophotometers, MtoZ Biolabs provides Host Cell Protein Assay Service for qualitative and quantitative analysis of HCP residues, which is applicable to the quality and safety assessment of a wide range of biopharmaceutical products.
Service Advantages
1. Advanced Analysis Platform: MtoZ Biolabs established an advanced host cell protein assay service platform, guaranteeing reliable, fast, and highly accurate analysis service.
2. One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
3. High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all host cell protein assay service data, providing clients with a comprehensive data report.
4. Multiple Detection Methods Available: In addition to the ELISA method, MtoZ Biolabs also offers 2D-DIGE (Two-Dimensional Difference Gel Electrophoresis) and mass spectrometry for host cell protein assays.
Sample Submission Suggestions
Sample Types
We accept a variety of sample types, including cell culture supernatants, purification intermediates, and final products such as recombinant proteins, antibodies, and vaccines.
Sample Quantity
The required sample quantity may vary depending on the sample type and experimental method. For example, bacterial pellets should be no less than 1 g, and cell pellets should contain at least 1×10⁷ cells. We encourage you to contact us for consultation before submitting your samples.
FAQ
Q: How to ensure that the antibodies used in ELISA cover as many HCPs as possible?
Ensuring that ELISA antibodies cover the widest range of HCPs is a critical aspect of developing effective HCP detection methods. The following strategies can be employed to optimize antibody coverage:
1. Use Polyclonal Antibodies
Polyclonal antibodies recognize multiple epitopes on antigens, providing broader HCP coverage compared to monoclonal antibodies. However, batch-to-batch variability in polyclonal antibodies may occur, requiring strict production and quality control measures.
2. Employ Immunogen Mixtures
During antibody preparation, use HCP mixtures from specific production stages, such as cell lysates or early-stage purification samples, as immunogens. This approach ensures that the antibodies reflect the diversity of HCPs present in the expression system.
3. Cross-Validation with Mass Spectrometry
Validate antibody coverage using mass spectrometry (MS). MS can identify HCPs that are not detected by ELISA, providing valuable feedback for assessing and optimizing antibody selection.
4. Supplement Antibody Libraries
When antibody coverage is insufficient, develop new polyclonal or monoclonal antibodies to supplement the existing library. This enhances detection capabilities, particularly for low-abundance or unique HCPs.
5. Choose Commercial ELISA Kits
For common expression systems (e.g., CHO, HEK293), opt for validated commercial HCP ELISA kits. These kits typically offer high coverage and meet regulatory requirements, providing reliable and efficient solutions.
By combining these strategies, it is possible to maximize the coverage of HCPs in ELISA assays, ensuring accurate and comprehensive detection to meet regulatory and quality standards.
Case Study
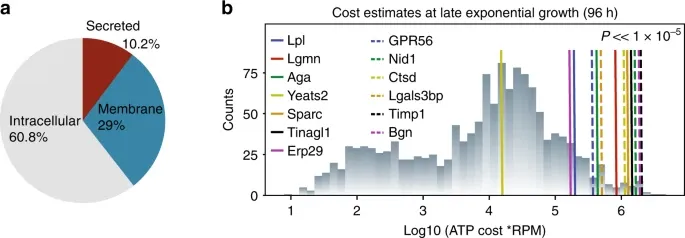
This study utilized host cell protein assay to analyze gene-edited host cells, revealing that targeted regulation of the host cell secretion pathway can significantly reduce HCP levels, improve the purity of the target recombinant protein, and enhance production efficiency.
Kol S. et al. Nat Commun. 2020.
How to order?







