Host Cell Protein (HCP) Analysis
- High Sensitivity and Precision: HPLC-MS/MS technology provides highly sensitive and accurate measurements, capable of detecting low-abundance HCPs.
- Broad Applicability: Suitable for various biological samples, including cell culture supernatants, purified products, and final biopharmaceuticals.
- Rapid and Efficient: Optimized sample preparation and fast analysis ensure quick and efficient processing.
- Accurate and Reliable Data: Ensures high reproducibility and low error rates, supporting quality control in biopharmaceutical development and production, providing reliable and specific results.
Host cell proteins (HCPs) are non-target proteins produced by host cells, such as bacteria, yeast, insect cells, or mammalian cells, during biopharmaceutical production. These proteins may remain in the final products after cell lysis and purification processes, potentially triggering immune responses, reducing drug stability and efficacy, and affecting safety. Therefore, detecting and monitoring HCPs is critical in biopharmaceutical development and production to ensure their concentrations meet regulatory standards for safety and efficacy.
HCPs come from various sources, including structural proteins, enzymes, transport proteins, and other functional proteins of the host cells. Different host systems produce varying types and quantities of HCPs, impacting detection methods and difficulty. Accurate identification and quantification of HCPs are essential for optimizing production processes, improving product purity, and ensuring patient safety.
MtoZ Biolabs uses high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) for HCP analysis. This advanced technology combines efficient HPLC separation with sensitive MS/MS detection, ensuring accurate identification and quantification of various HCPs in complex biological samples.
Analysis Workflow
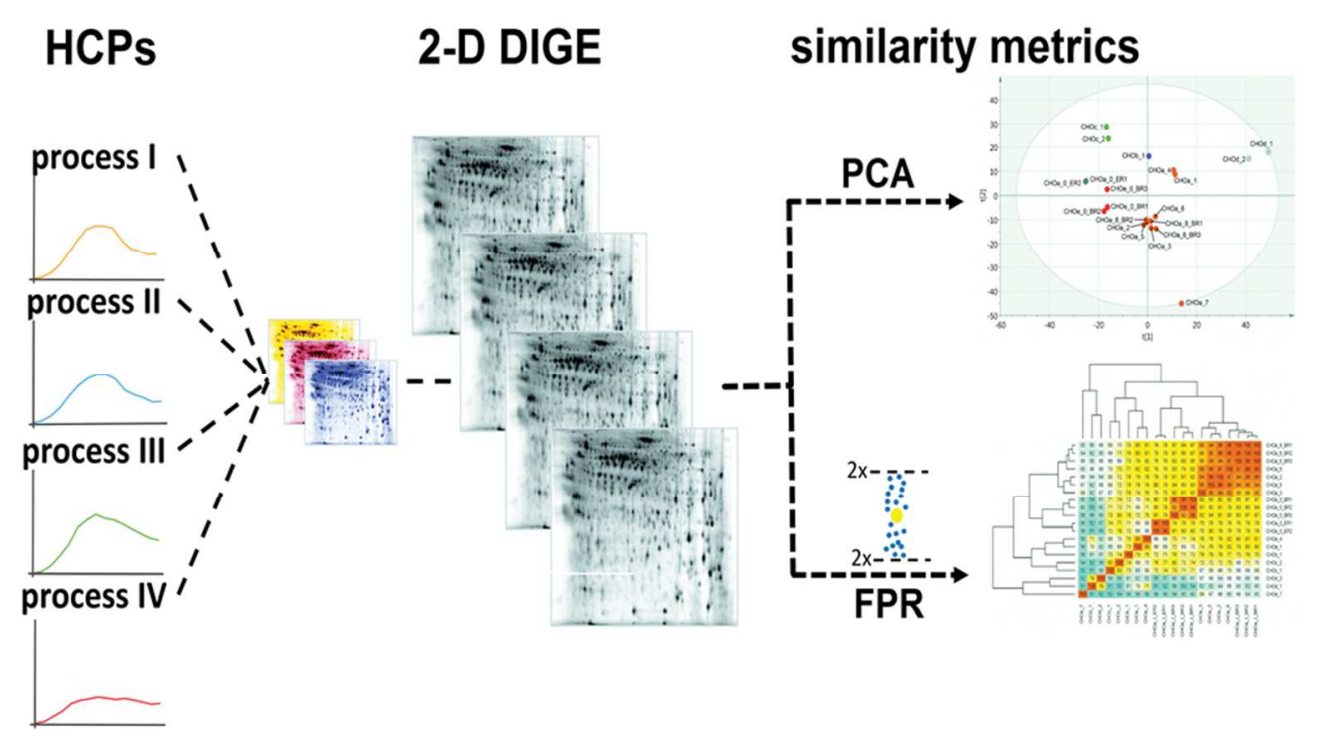
Obrstar, D. et al. Anal. Chem. 2018.
Service Advantages
Sample Submission Requirements
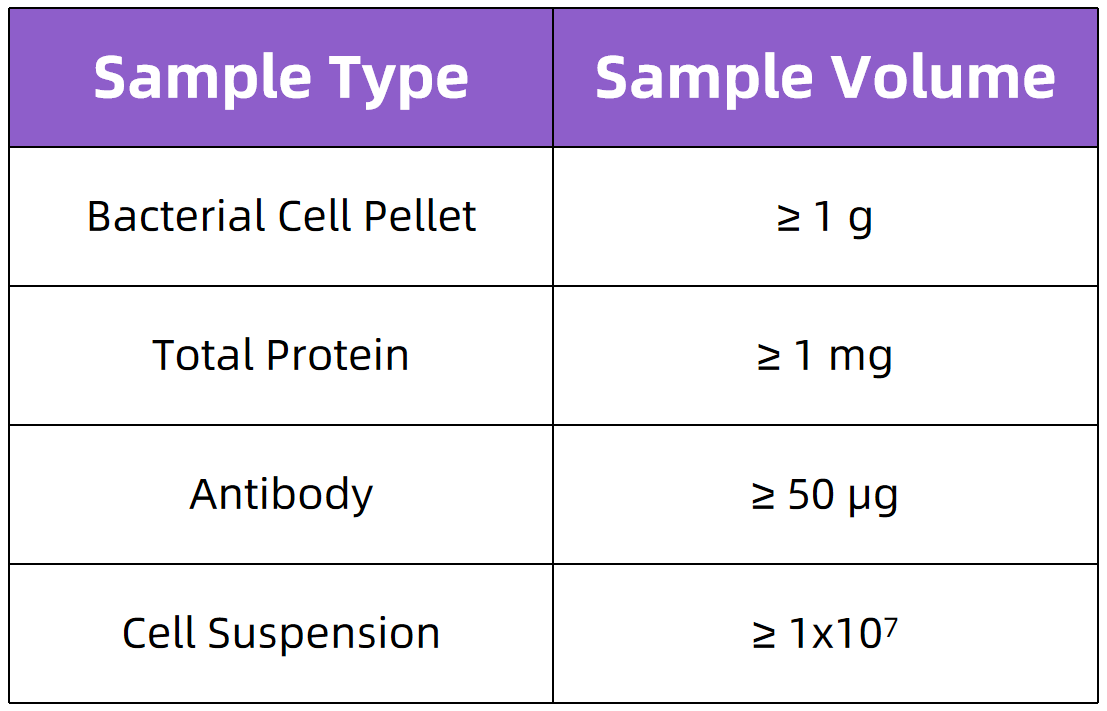
For detailed sample submission instructions, please consult our technical team.
Applications
Biopharmaceutical Development: Monitor and detect HCPs during drug development to ensure the purity and safety of biopharmaceutical products.
Process Control: Regularly detect HCPs during production to optimize processes and reduce impurity levels.
Quality Control: Detect HCPs in final products to ensure biopharmaceuticals meet regulatory requirements and quality standards.
Regulatory Submission: Provide accurate HCP data to support biopharmaceutical companies in meeting regulatory submission requirements.
How to order?







