Hybridoma Antibody Sequencing Service
- Sample Type: Total RNA, Frozen or live hybridoma cells.
- Details regarding the antibody isotypes.
- Information about the species and origins of the hybridoma cells.
Hybridoma technology has been a cornerstone in the development of monoclonal antibodies, offering the ability to generate highly specific antibodies for research, diagnostics, and therapeutic applications. However, to fully leverage the potential of hybridoma-derived antibodies, it is essential to obtain the underlying genetic information that defines their structure and specificity. Hybridoma Antibody Sequencing Service addresses this need by extracting the antibody genes from hybridoma cells and sequencing the heavy and light chain variable regions to uncover the genetic code responsible for the antibody's unique properties.
Hybridoma cells, while a powerful tool for monoclonal antibody production, are highly sensitive to contamination and suboptimal culture conditions, which can result in the loss of critical antibody genes over time. Without proper preservation, the unique genetic sequence that encodes the antibody may be lost, making it difficult to regenerate or reproduce the antibody in the future. This underscores the importance of sequencing hybridoma-derived antibody genes early in the research or development process. By obtaining the antibody gene sequence, researchers can not only preserve the genetic information for future use but also enable recombinant antibody expression, facilitating the long-term production and scalability of antibodies for therapeutic or diagnostic applications.
Services at MtoZ Biolabs
MtoZ Biolabs, an integrated Chromatography and Mass Spectrometry (MS) Services Provider, provides advanced proteomics, metabolomics, and biopharmaceutical analysis services to researchers in biochemistry, biotechnology, and biopharmaceutical fields. Our ultimate aim is to provide more rapid, high-throughput, and cost-effective analysis, with exceptional data quality and minimal sample consumption.
At MtoZ Biolabs, we provide a comprehensive Hybridoma Antibody Sequencing Service to meet these needs. Our service utilizes advanced molecular biology techniques, including mRNA extraction from hybridoma cells, cDNA synthesis, and PCR amplification, followed by high-fidelity sequencing to obtain the full antibody gene sequence. This allows us to provide researchers and biotechnology companies with the precise genetic information required for efficient antibody engineering and the development of novel therapeutic antibodies.
Analysis Workflow
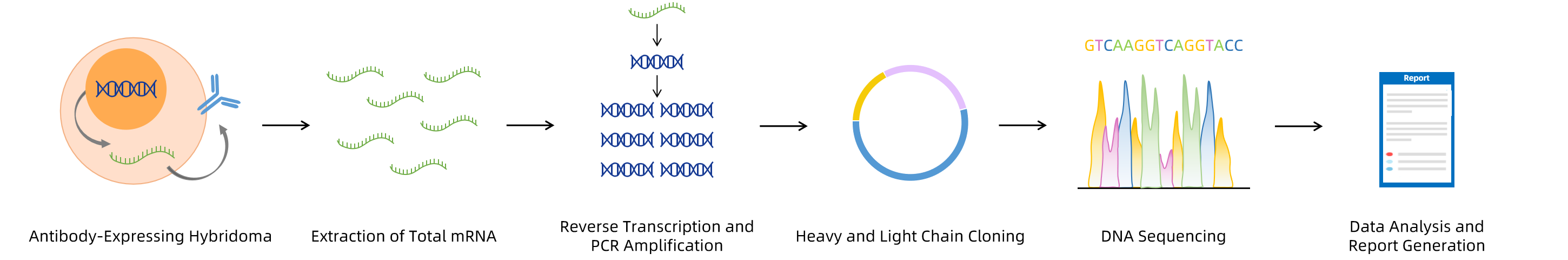
Why Choose MtoZ Biolabs?
1. Advance Analysis Platform
MtoZ Biolabs established an advanced Hybridoma Antibody Sequencing Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
2. One-Time-Charge
Our pricing is transparent, no hidden fees or additional costs.
3. High-Data-Quality
Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrate all Hybridoma Antibody Sequencing data providing clients with a comprehensive data report.
4. Extensive Expertise
Leveraging our expertise in primer design and antibody gene analysis, we provide highly efficient and accurate sequencing results.
5. Versatile Sequencing Services
Our service offers highly versatile sequencing capabilities, revealing both the variable region and full-length antibody protein sequences, ensuring comprehensive insights into your antibodies' genetic makeup.
6. Wide Applicability
Our service supports antibody gene sequencing across various species (mouse, rat, etc.) and isotypes (IgG1, IgG2a, IgG2b, IgM, etc.), ensuring broad applicability for diverse research needs.
Applications
✅ Patent Application
The unique amino acid sequence of an antibody, particularly the variable regions, is critical for establishing novelty and securing intellectual property. By sequencing the antibody's genetic code, you can clearly distinguish your antibody from others, ensuring exclusive patent rights for downstream developments and applications.
✅ Recombinant Antibody Expression
With access to the complete antibody sequence, you can efficiently develop recombinant antibodies for therapeutic or research purposes. This also allows for the conversion of antibodies into different isotypes and formats, or the expression of antibodies in various systems (e.g., bacterial, mammalian), ensuring scalable and consistent production.
✅ Antibody Downstream Development
Beyond recombinant expression, the antibody sequence serves as a blueprint for various downstream developments, such as humanization, affinity maturation, and the creation of bispecific antibodies. It also facilitates hybridoma cell line validation and format alteration, ensuring flexible and efficient research pathways.
✅ Protection Against Cell Line Loss
In case of hybridoma cell line loss due to contamination, improper handling, or freezer breakdown, sequencing provides a backup. The antibody gene sequence ensures that recombinant antibodies can still be produced, preventing disruptions in research or production.
Sample Submission Suggestions
*Note: Feel free to contact us if you have any additional sample requirements or would like more detailed information regarding sample submission.
FAQ
Q1: Can hybridoma sequencing be used for any type of antibody?
Yes, hybridoma sequencing can be applied to any antibody produced by hybridoma cells. It is a versatile technique suitable for sequencing both monoclonal and polyclonal antibodies. However, due to the nature of hybridoma technology, it is primarily used for monoclonal antibodies that produce a single type of antibody molecule.
Q2: What challenges might be encountered during the hybridoma sequencing process?
Challenges may include:
1. Low mRNA abundance, which can impair cDNA synthesis
2. PCR amplification errors or biases, particularly in regions rich in GC content or with complex structures
3. Sequencing errors or artifacts
To overcome these obstacles, it's essential to optimize sample preparation methods, carefully design PCR primers, and employ high-fidelity enzymes along with dependable sequencing platforms.
Deliverables
1. Experimental Procedures
2. DNA Sequence Alignments of Heavy and Light Chain Variable Regions
3. DNA Gel Images
4. Analytical Raw Data Files
5. Sequencing Results
How to order?







