Medical Device III Special Analysis Service
Class III medical devices are medical devices that pose potential danger to the human body and require strict control to ensure their safety and effectiveness. These devices typically include implants, life-supporting or life-sustaining devices, and equipment that poses potential danger to the human body.
Testing Content Required by Policies and Regulations (United States)
1. Characterization of Type I Collagen as Starting Material for Surgical Implants and Substrates for Tissue Engineered Medical Products (TEMPs)
Summary of the Standard Approval for Recombinant Collagen Products: ASTM F2212-20
(1) Scope
This guide aims to provide manufacturers, producers, and researchers with information on the characteristics, properties, and test methods of collagen to more clearly identify collagen-containing biomaterials used. It focuses on the characterization of Type I collagen, the most abundant collagen in mammals, especially in skin and bone. This guide does not provide specific parameters for any collagen products or product combinations, nor does it address the acceptability of these products for their intended use. The collagen may be from any source including, including but not limited to animal or cadaveric sources, human cell culture, or recombinant sources.
(2) Requirements for the Purification Process of Collagen Materials
① Impurity Removal: Remove impurities such as non-collagenous proteins, lipids, carbohydrates, and cell debris that may be present in the raw materials.
② Endotoxin Levels: Ensure that the endotoxin levels in collagen products are below the safety threshold to avoid triggering an immune response.
③ Pathogen Removal: Ensure that collagen products are free of viral, bacterial, and other pathogenic contamination through appropriate purification steps.
④ Protein Integrity: Maintain the triple-helix structure of collagen molecules and avoid excessive degradation during purification.
⑤ Host Cell Protein (HCP) Residue: If collagen is produced in host cells using recombinant technology, minimize the residual HCP.
⑥ Residual DNA: Ensure that residual DNA from the host cell source is effectively removed.
⑦ Uniformity and Consistency: Ensure that each batch of collagen has consistent quality and attributes.
⑧ Stability: Ensure that collagen remains stable during storage and use, without significant degradation or structural changes.
⑨ Functional Testing: Conduct necessary functional tests to demonstrate that purified collagen retains its biological functions.
⑩ Detailed Documentation: Record the detailed purification process, including chemicals used, process parameters, test results, and quality control measures.
(3) Specific Requirements for the Safety and Biocompatibility of Recombinant Collagen Products Approved by ASTM F2212-20
① Material Characteristics and Characterization: Detailed description and characterization of collagen materials to ensure they meet specific property and performance requirements.
② Biocompatibility: The product must have good biocompatibility, usually demonstrated through historical clinical use and laboratory studies.
③ Material Source: Collagen can come from different sources, including animals, cadavers, human cell culture, or recombinant sources. Each source's collagen may have different biological, immunological, and toxicological properties and should be thoroughly studied according to its origin.
④ Purification Process: As a starting material for surgical implants and tissue engineered medical products (TEMPs), Type I collagen must be purified to reduce impurities or contaminants that may cause adverse reactions.
⑤ Test Methods: The standard provides test methods for evaluating collagen characteristics and properties, which may include mechanical testing, chemical analysis, and biocompatibility testing.
⑥ Manufacturer's Responsibility: The manufacturer is responsible for ensuring the biocompatibility and applicability of their products for specific applications.
⑦ Safety Warning: The standard also includes a safety warning about mercury, noting that mercury is a harmful substance and appropriate safety measures should be taken when handling it.
⑧ Safety and Health Practices: Users of the standard are responsible for establishing appropriate safety, health, and environmental practices and determining the applicability of regulatory restrictions prior to use.
⑨ SI Units: The values used in the standard are in international system of units (SI), which helps ensure global consistency and comparability.
⑩ Regulatory Compliance: Products must comply with all relevant national and international regulatory requirements.
Tissue Engineering Products in Class III Medical Devices Quality Analysis Solutions
Service Advantages
1. Professional and Reliable Services Covering Multiple Aspects of Recombinant Collagen Detection
2. Cost-Effective Testing, Short Experimental Cycles, and Comprehensive and Reliable Test Reports
3. Equipped with Various High-Resolution Mass Spectrometers and Other Large Instruments
Case Study
1. Production of Recombinant Human Procollagen Type I C-terminal Propeptide and Establishment of a Sandwich ELISA for Quantification
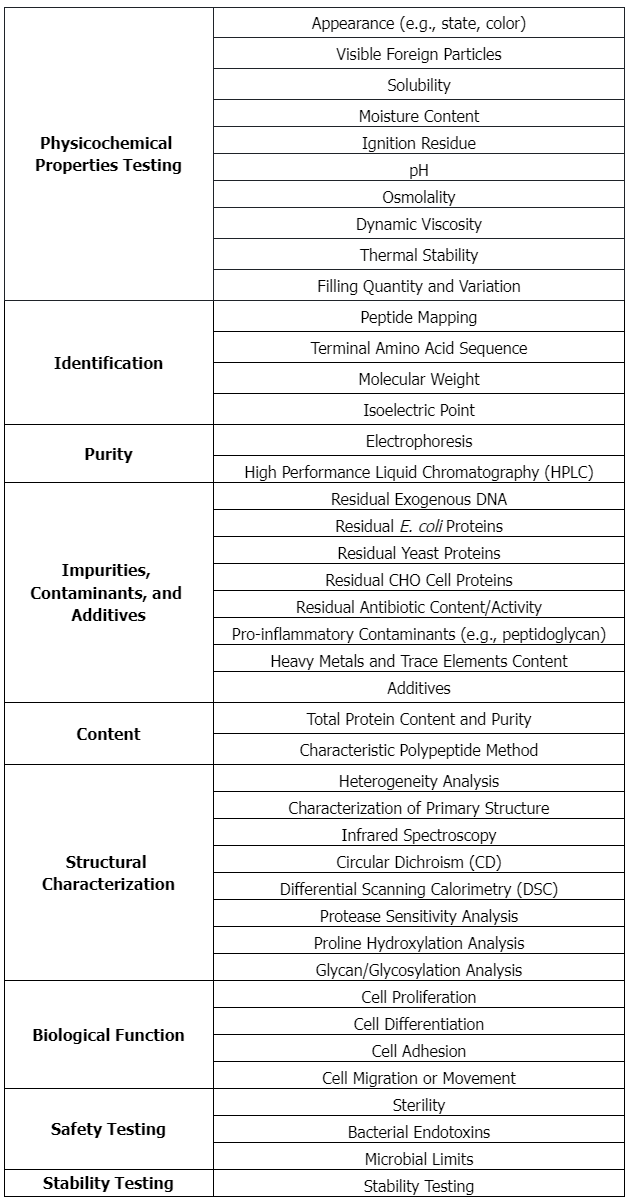
Procollagen Type I carboxy-terminal propeptide (PICP), derived from Type I procollagen, has been identified as an indicator of Type I collagen synthesis in bone matrix formation and skin recovery. PICP is a heterotrimeric glycoprotein composed of two α1 chains (PICPα1) and one α2 chain (PICPα2). Studies have reported the recombinant expression of human PICP using a mammalian expression system. Co-expression of PICPα1 and PICPα2 in HEK293F cells resulted in the production of functional PICP in a correctly assembled heterotrimeric form. Using recombinant PICP as an antigen, researchers isolated PICP-specific human monoclonal antibodies from a phage display antibody library and elevated rabbit polyclonal antibodies. With these antibodies, the researchers developed a sandwich ELISA for PICP with a detection limit of 1 ng/mL and a measurable range of 1–640 ng/mL. Intra- and inter-assay imprecision values were both <10%. To measure PICP levels in human fibroblast extracts, culture supernatants, and human serum, the developed ELISA kit showed performance comparable to that of a commercial kit. The study results provided an effective production strategy for recombinant PICP, promoted the production of PICP-specific antibodies, and facilitated the development of the PICP sandwich ELISA with potential applications in the clinical diagnosis of serum samples and the detection of cosmeceutical ingredients in fibroblast cultures.
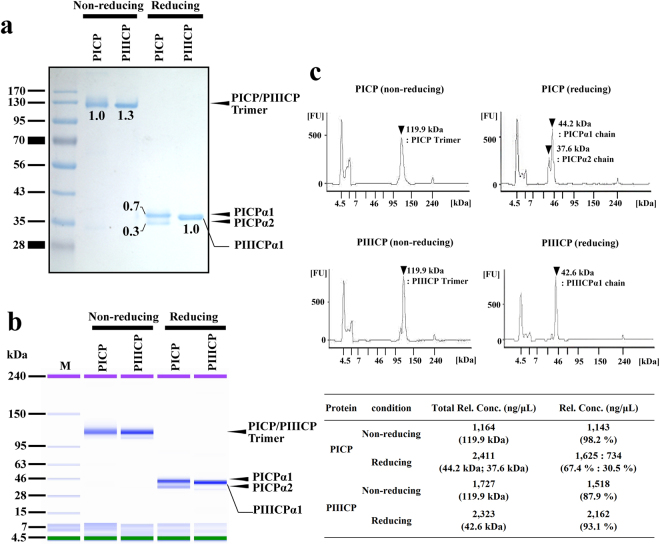
Figure 1. SDS-PAGE and SDS-CGE Analysis of Purified Recombinant PICP and PIICP [1]
2. Quantitative Analysis of Denatured Collagen by Collagenase Digestion Followed by MALDI-TOF Mass Spectrometry
Collagen is the most abundant protein in vertebrate tissues and an important component of the extracellular matrix (ECM). The determination of collagen content is relevant not only in the field of native tissue research but also for evaluating the quality of bioengineered tissues. A study described a quantitative method for evaluating small amounts of collagen based on MALDI-TOF (matrix-assisted laser desorption/ionization time-of-flight) mass spectrometry (MS) after digesting the collagen with clostridial collagenase (clostridiopeptidase A) to obtain characteristic oligopeptides. Among the obtained peptides, Gly-Pro-Hyp, which strongly indicates collagen, was used to assess collagen content by comparing the intensity of the Gly-Pro-Hyp peak to that of a spiked tripeptide (Arg-Gly-Asp). The method presented in this paper was simple and convenient and allowed for the determination of collagen in microgram amounts. In tissue samples such as cartilage, the actual collagen content was also determined by nuclear magnetic resonance (NMR) spectroscopy after acidic hydrolysis for comparison. Both methods provided consistent data within ±10% experimental error. Although this method cannot distinguish different types of collagen, it can easily determine the overall collagen content of tissues.
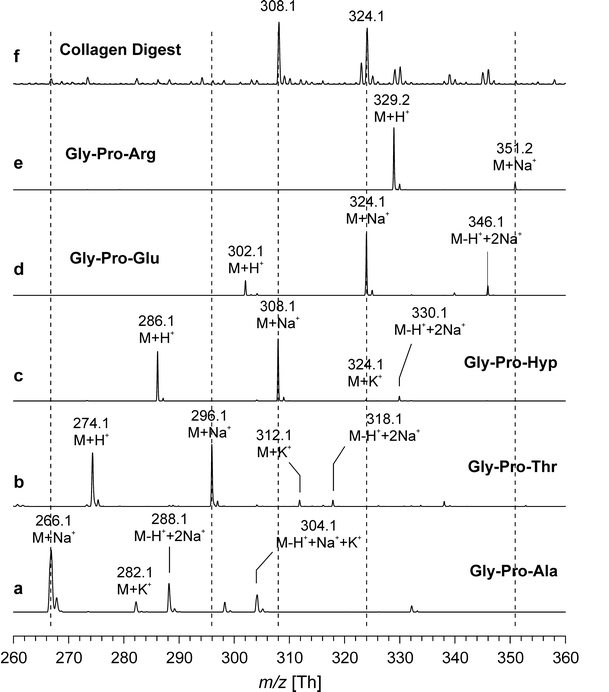
Figure 2. Positive Ion MALDI-TOF Mass Spectra of Synthetic Peptides and Enzymatic Digestion Products [2]
3. Determination of Proline Hydroxylation in Recombinant Collagen Variants by Liquid Chromatography-Mass Spectrometry
The preparation of recombinant collagen proteins and their prescribed variants have significant potential for tissue regeneration, cell-matrix interaction research, and fundamental biochemical and biophysical studies of the ECM. Recombinant expression requires proline hydroxylation, a post-translational modification crucial for imparting stability and structure. However, these modifications are not native to typical bacterial or yeast expression systems. Furthermore, detecting low levels of 4-hydroxyproline is challenging in terms of selectivity and sensitivity. A study developed a novel liquid chromatography-mass spectrometry (LC-MS) method to assess proline hydroxylation in recombinant collagen. This assay was tested in various Saccharomyces cerevisiae expression systems to evaluate the effect of gene ratio between prolyl-4-hydroxylase and collagen on hydroxylation contents. These systems used the human collagen III gene synthesized de novo from oligonucleotides. LC-MS detection did not require derivatization, used picomoles of samples, and could measure proline hydroxylation levels in recombinant and natural collagen proteins, ranging from approximately 0% to 40%. Hydroxylation values obtained by LC-MS were as accurate as those obtained by conventional amino acid analysis methods. A simple, derivatization-free LC-MS method was developed to accurately determine the percentage of proline hydroxylation in different yeast expression systems. Using this assay, researchers found that systems with a higher collagen-to-hydroxylase gene copy ratio produced a lower percentage of hydroxylation, indicating that a specific balanced gene ratio was required to achieve higher hydroxylation levels.
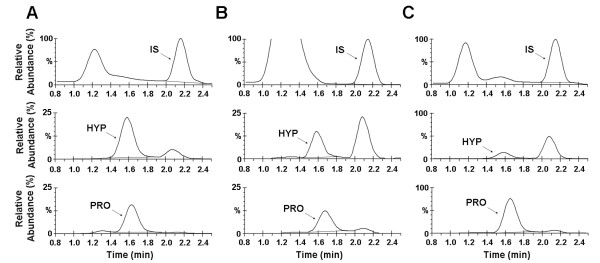
Figure 3. Reconstructed Ion Chromatograms of Hydrolyzed Collagen Samples [3]
References
[1] Seo WY, Kim JH, Baek DS, Kim SJ, Kang S, Yang WS, Song JA, Lee MS, Kim S, Kim YS. Production of recombinant human procollagen type I C-terminal propeptide and establishment of a sandwich ELISA for quantification. Sci Rep. 2017 Nov 21;7(1):15946. doi: 10.1038/s41598-017-16290-9. PMID: 29162919; PMCID: PMC5698462.
[2] Nimptsch A, Schibur S, Ihling C, Sinz A, Riemer T, Huster D, Schiller J. Quantitative analysis of denatured collagen by collagenase digestion and subsequent MALDI-TOF mass spectrometry. Cell Tissue Res. 2011 Mar;343(3):605-17. doi: 10.1007/s00441-010-1113-2. Epub 2011 Jan 29. PMID: 21274570.
[3] Chan SW, Greaves J, Da Silva NA, Wang SW. Assaying proline hydroxylation in recombinant collagen variants by liquid chromatography-mass spectrometry. BMC Biotechnol. 2012 Aug 17;12:51. doi: 10.1186/1472-6750-12-51. PMID: 22901055; PMCID: PMC3443662.
How to order?







