HILIC-UHPLC Based N-Glycosidic Bonds Analysis Service
Glycosidic bonds rarely affect the function of glycoproteins, thus they are usually not necessary to determine. However, the glycan structure of glycoproteins is highly complex, and assessing the configuration and location of N-glycan glycosidic bonds is helpful for analyzing the structure of glycoproteins. There are five common types of N-glycosidic bonds, with the most common being the connection between N-acetylglucosamine (GlcNAcβ1-Asn) and asparagine (Asn). Other types connected to Asn include glucose in mammalian and archaebacterial calnexins, N-acetylgalactosamine (GalNAc) in archaebacteria, and rhamnose in bacteria. It has also been reported that glucose is connected to arginine in sweet corn.
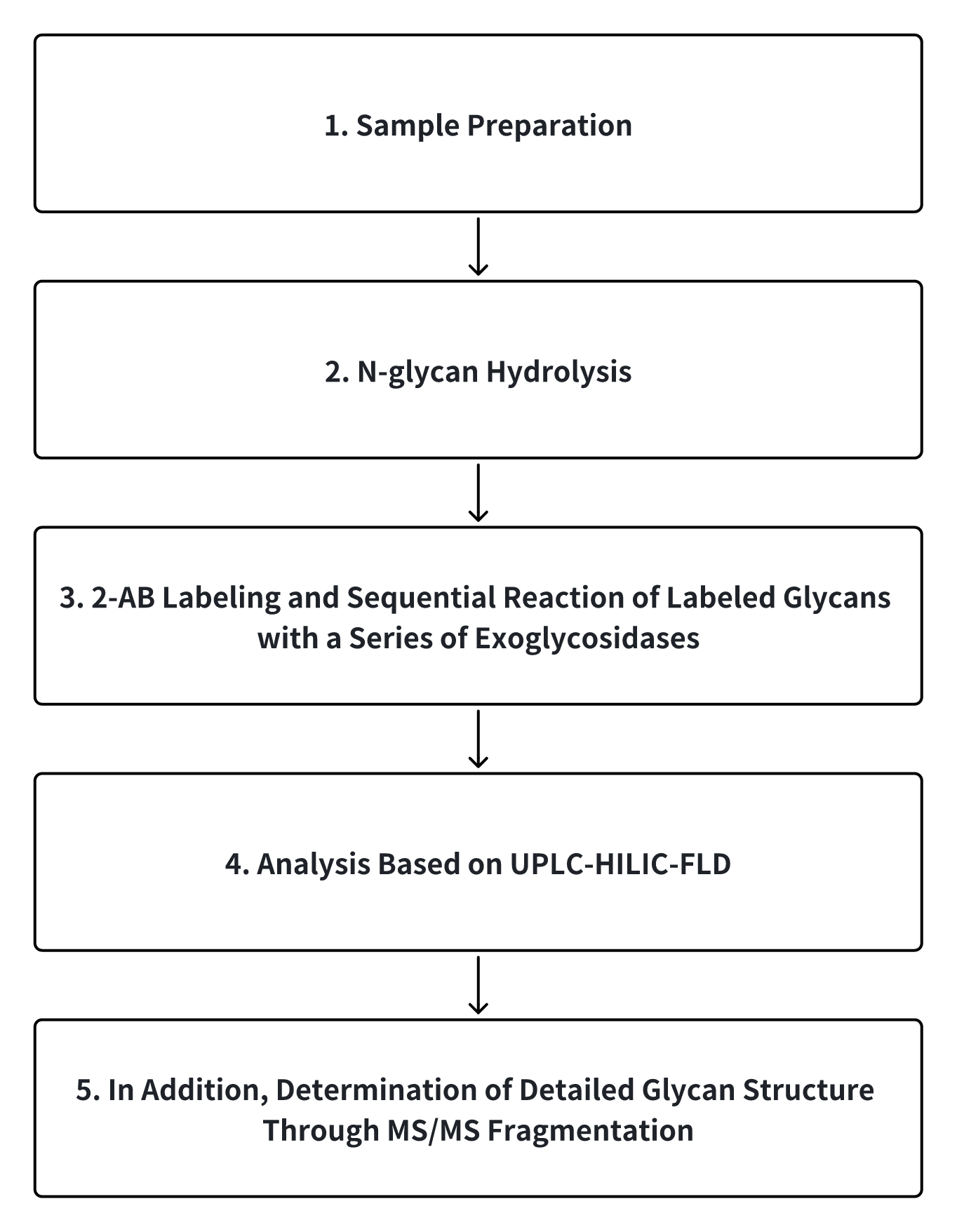
N-glycans are separated from glycoproteins through hydrolyzation by peptide N-glycosidase F (PNGase F). The separated N-glycans are labeled with 2-aminobenzamide (2-AB) and then digested with various exoglycosidases. Finally, the N-glycans are analyzed by hydrophilic interaction chromatography (HILIC) combined with fluorescence and MS.
Analysis Workflow
1. Role of Endoglycosidases
Endoglycosidases hydrolyze oligosaccharides from glycoproteins or glycolipids. Usually, they do not hydrolyze oligosaccharides from the bound protein and lipid molecules. Endoglycosidases function by breaking the glycosidic bond between two monosaccharides in a polymer.
(1) Endoglycosidase H
Hydrolyzes oligomannose and hybrid N-glycans.
(2) Endoglycosidase F
Hydrolyzes simple divalent N-glycans.
How to order?







