Natural Diterpene DA: The "Powerful Tool" from Wikstroemia chamaedaphne for Anti-Renal Fibrosis
Renal fibrosis is a hallmark pathological feature of chronic kidney disease (CKD), characterized by excessive deposition of extracellular matrix (ECM) in the renal interstitium and sustained activation of fibroblasts, which critically impair renal function and reduce patient quality of life. Among various profibrotic factors, transforming growth factor-β1 (TGF-β1) plays a central role in regulating renal fibrosis. Consequently, targeting TGF-β1 and its downstream signaling pathways has become a promising therapeutic strategy for CKD. Traditional herbal medicine has long been used to manage fibrotic diseases, yet the active components and underlying mechanisms of many remedies remain poorly understood. Wikstroemia chamaedaphne, a plant rich in antifibrotic natural products, has been used in traditional medicine to treat edema, a common manifestation of kidney disorders. Recent studies have shown that crude extracts (CE) from W. chamaedaphne significantly mitigate renal fibrosis. Further isolation led to the identification of compound 15, named dapnepeduin A (DA), which attenuates renal fibrosis through modulation of the Cdc42-mediated GSK-3β/β-catenin signaling pathway. These findings emphasize the potential of DA as a natural small-molecule antifibrotic agent, providing a promising direction for future therapeutic strategies targeting renal fibrosis. This study provides a foundation for further exploration of the mechanisms behind DA's antifibrotic effects. The following sections highlight the key experimental methods, findings, and molecular insights that led to the identification of DA, along with its potential implications for novel therapeutic approaches to CKD.
1. Discovery of DA as an Effective Inhibitor of Renal Fibroblast Activation
The crude extract (CE) of W. chamaedaphne significantly reduced renal fibrosis in mice, as shown in Figure 1A. In vitro experiments demonstrated that TGF-β1 consistently induced the expression of α-SMA and ECM production in renal fibroblasts. However, CE, at concentrations starting from 5 µg/mL, inhibited these markers in a dose-dependent manner, suggesting its potential in suppressing fibroblast activation (Figure 1B).
To identify the active components in CE, the extract was fractionated, leading to the isolation of 16 diterpenoids (compounds 1-16). Among these, compound 15 (dapnepeduin A, DA) displayed the strongest antifibrotic activity. Specifically, DA effectively reduced fibronectin and type I collagen production, as shown in Figure 1D. Additionally, in MTS assays, DA demonstrated low cytotoxicity to fibroblasts, with >90% cell viability maintained at 10 µM. Considering its efficacy and safety profile, DA was selected as a lead compound for further analysis. Subsequent studies revealed that DA reduced fibrosis marker expression in both dose- and time-dependent manners (Figures 1E, F).
In conclusion, DA, a primary component of W. chamaedaphne, was identified as a potent inhibitor of renal fibroblast activation and ECM production.
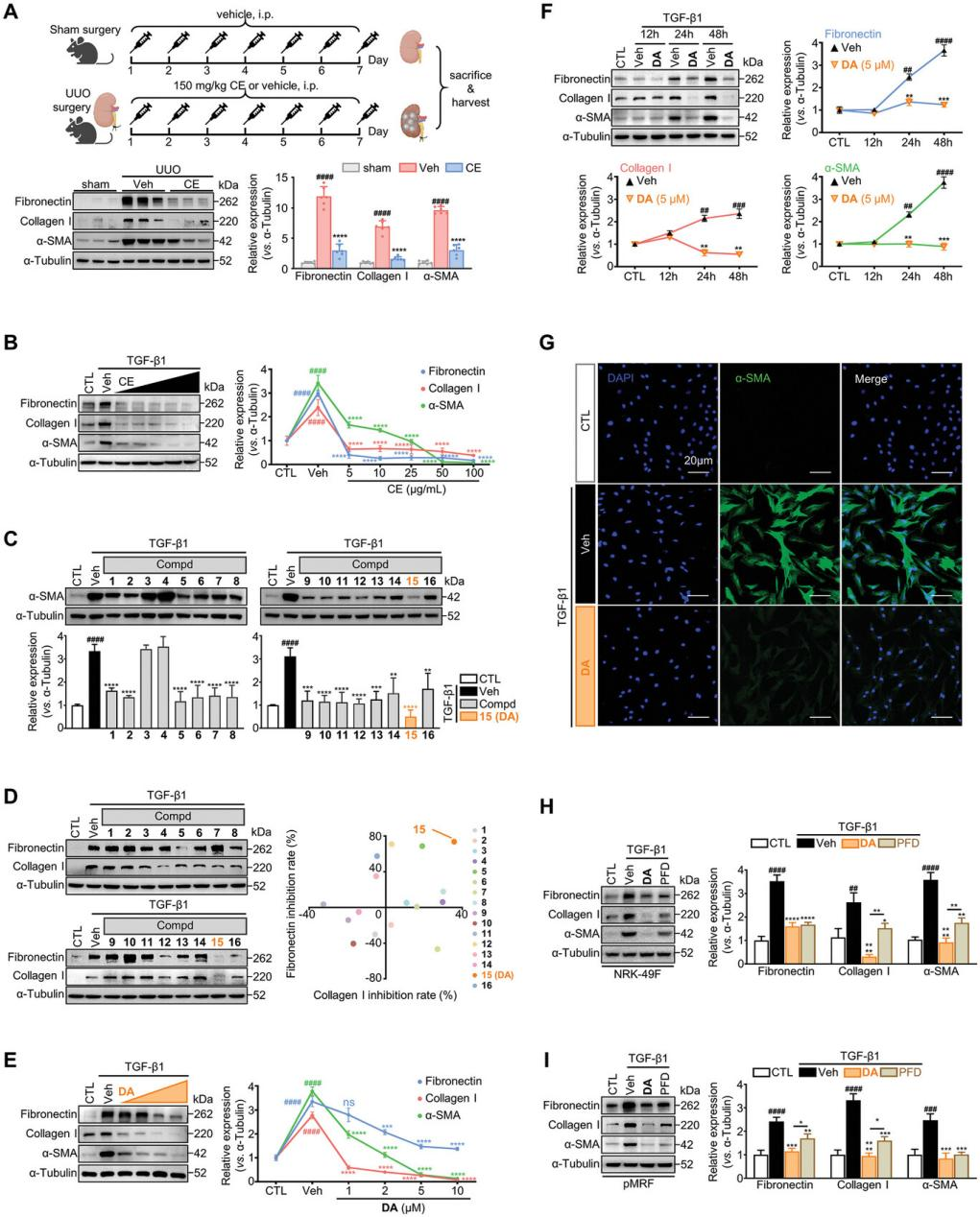
Figure 1. DA as an effective antifibrotic compound in TGF-β1-activated renal fibroblasts.
2. DA Inhibits Epithelial-Mesenchymal Transition and Renal Fibroblast Activation
Activated renal fibroblasts exhibit increased proliferation and migration, driving the progression of renal fibrosis. The study then assessed the effects of DA on activated fibroblasts. In fibroblasts activated by TGF-β1, angiotensin II, IL-17, LPS, and IL-1β, DA suppressed α-SMA and ECM expression and inhibited fibroblast proliferation and migration. Moreover, DA mitigated epithelial-mesenchymal transition (EMT), a process crucial to fibrosis progression. Specifically, DA restored the expression of the epithelial marker E-cadherin and reduced the levels of mesenchymal markers, including fibronectin and vimentin. These findings highlight the significant antifibrotic potential of DA.
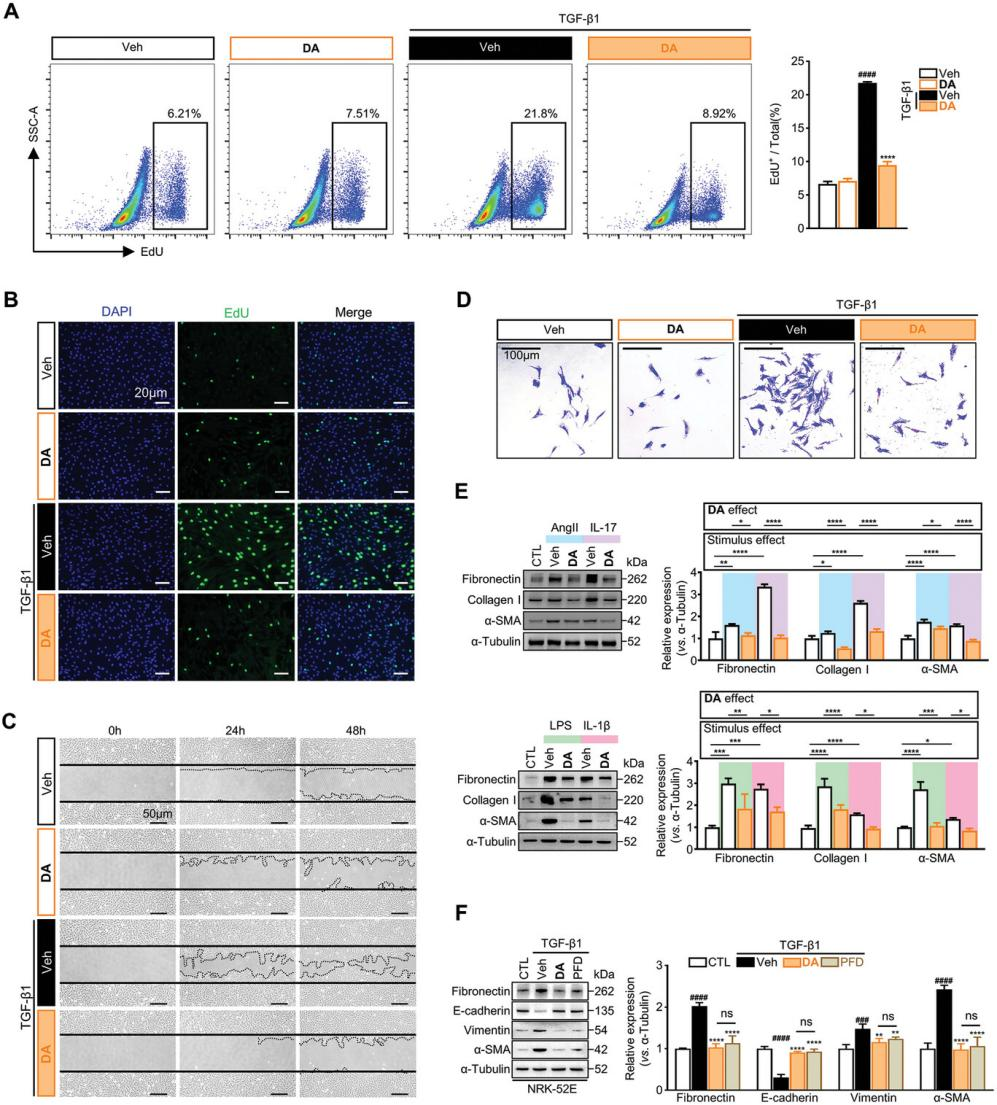
Figure 2. DA blocks renal fibroblast activation and EMT in tubular epithelial cells.
3. DA Alleviates Renal Fibrosis in a UUO Mouse Model
The in vivo antifibrotic efficacy of DA was evaluated using a UUO mouse model. Low-dose (LD) and high-dose (HD) DA were administered seven days post-surgery. DA treatment significantly reduced the mRNA and protein levels of Acta2 (encoding α-SMA), Col1a1, Col3a1 (encoding collagens), and Fn1 (encoding fibronectin) compared with the control group (Figures 3A and 3B). The LD group exhibited similar efficacy to the PFD group (positive control) in reducing these fibrotic markers, while the HD group demonstrated superior efficacy, highlighting the potent antifibrotic properties of DA. Importantly, DA administration did not induce systemic toxicity, as evidenced by stable serum AST and ALT levels and no significant changes in body weight (Figures 3D and 3E).
HPLC-MS/MS analysis revealed DA’s organ distribution, a critical factor for therapeutic efficacy and safety. As shown in Figure 3F, DA was enriched in the kidney, with detectable levels in the liver, while no peaks were observed in the lung, heart, or spleen.
In conclusion, DA effectively mitigates tubular interstitial fibrosis in vivo, demonstrating both high efficacy and a favorable safety profile.
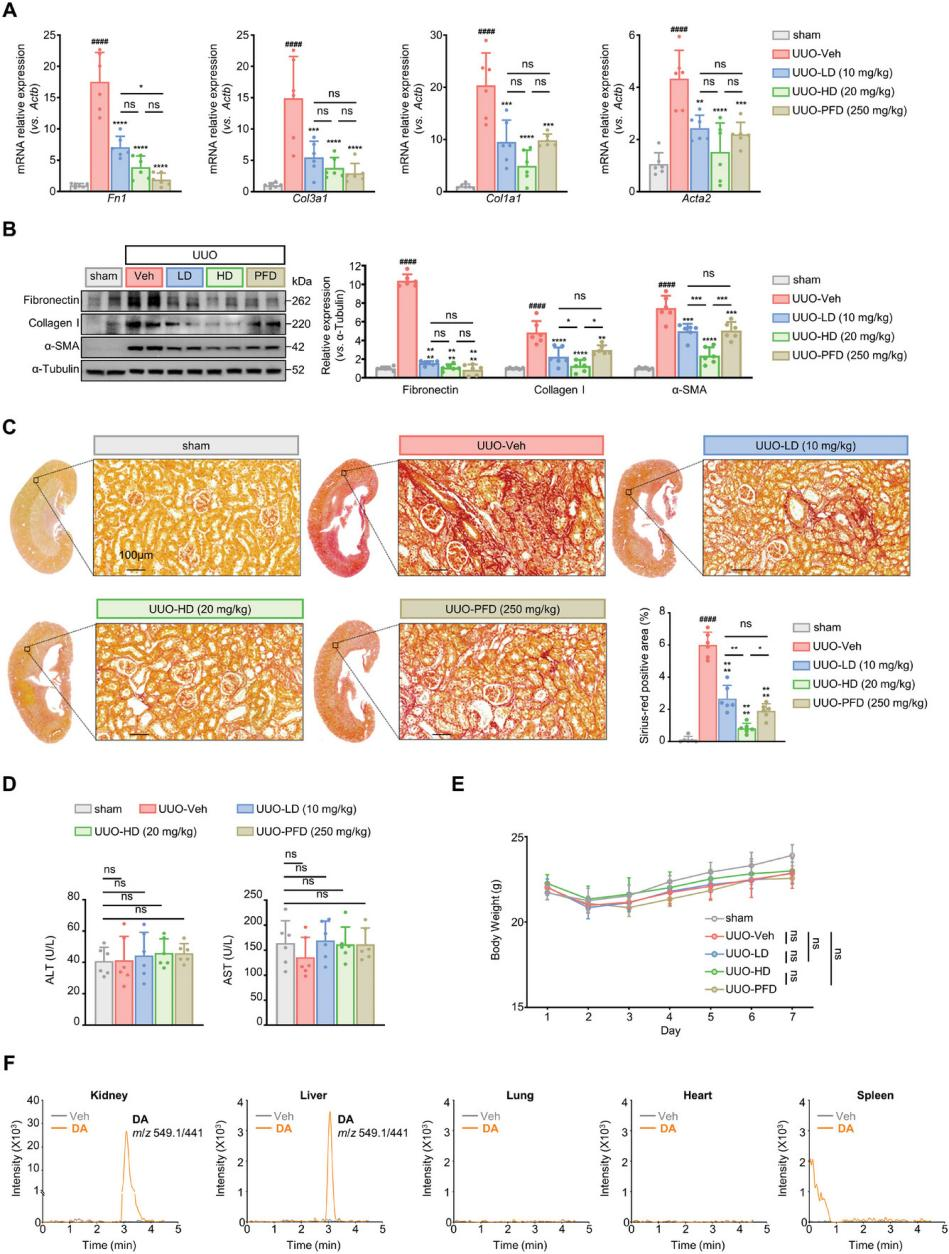
Figure 3. DA’s protective effects against renal fibrosis in a UUO mouse model.
4. DA Suppresses β-Catenin Accumulation to Inhibit Fibrosis
To elucidate DA’s molecular mechanism of action, transcriptomic analysis identified 1,593 differentially expressed genes, with 1,074 upregulated and 519 downregulated after DA treatment (Figure 4A). KEGG pathway analysis indicated that DA primarily modulated the Wnt signaling pathway rather than the profibrotic TGF-β/Smads pathway (Figure 4B). DA significantly reduced TGF-β1-induced β-catenin upregulation in both the cytoplasm and nucleus, which are hallmarks of Wnt/β-catenin signaling activation (Figures 4C–4E). Furthermore, DA consistently inhibited β-catenin levels across various renal fibroblast and tubular cell models, reinforcing its antifibrotic effects through Wnt pathway suppression.
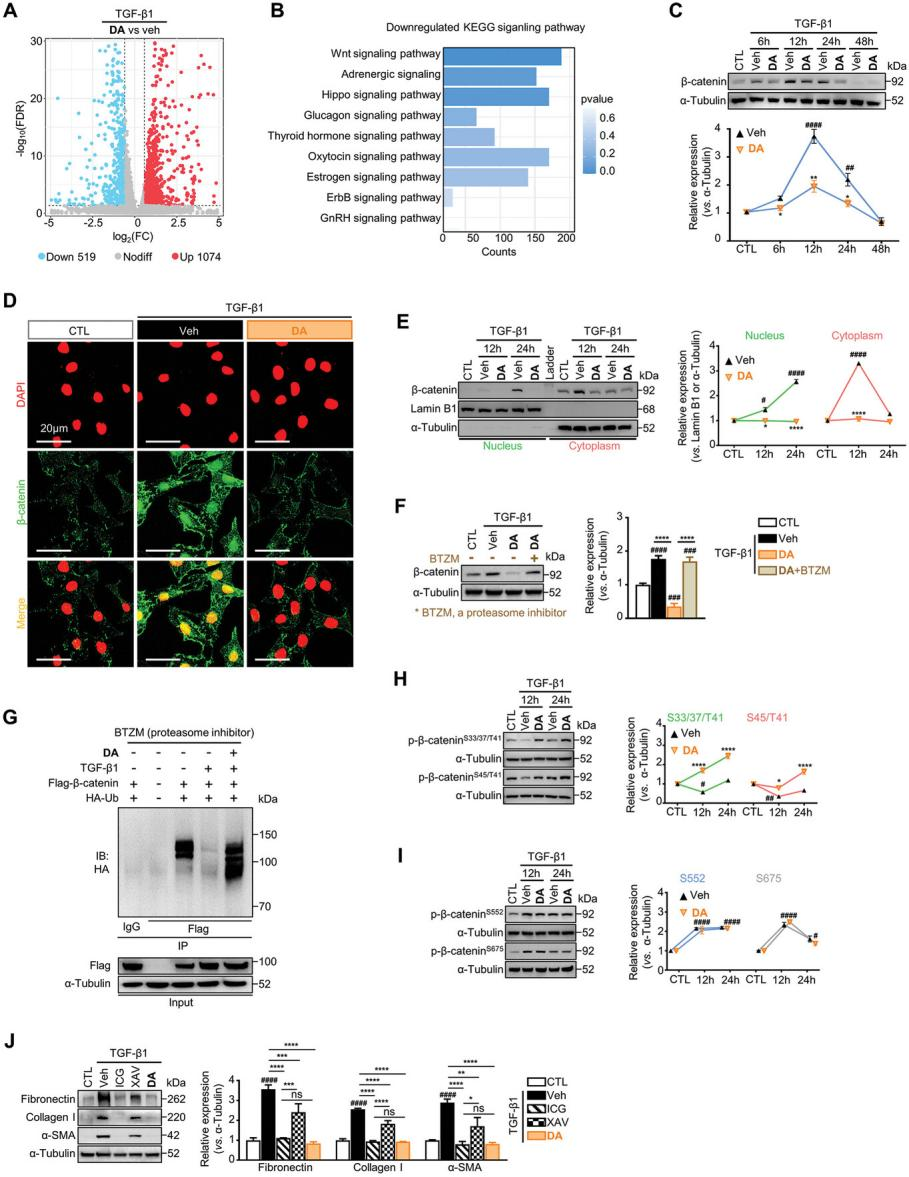
Figure 4. DA enhances β-catenin phosphorylation at S33/37/45/T41 residues and promotes its ubiquitination-mediated degradation.
DA upregulated β-catenin (Ctnnb1) mRNA levels in renal fibroblasts, suggesting that DA-mediated downregulation of β-catenin protein levels might result from enhanced degradation rather than transcriptional suppression. Validation experiments confirmed that DA promoted β-catenin hydrolysis (Figure 4F). To further investigate the mechanism, the effects of DA on β-catenin ubiquitination were analyzed. Results showed that DA treatment restored β-catenin ubiquitination, which had been reduced by TGF-β1 (Figure 4G). Ubiquitin-mediated proteolysis of β-catenin is tightly regulated by phosphorylation at key residues, including Ser33/37/45 and Thr41. Notably, TGF-β1 significantly reduced β-catenin phosphorylation at these sites, whereas DA antagonized this reduction, thereby facilitating β-catenin degradation (Figure 4H). Collectively, these findings indicate that DA enhances β-catenin ubiquitin-mediated degradation by promoting phosphorylation at Ser33/37/45 and Thr41, thereby suppressing its profibrotic signaling activity.
These results highlight that the antifibrotic effects of DA are primarily driven by its ability to block the profibrotic β-catenin signaling pathway.
5. DA Alleviates Renal Fibrosis in the UUO Mouse Model by Targeting β-Catenin Signaling
To determine whether DA modulates β-catenin signaling in vivo, the total β-catenin and phosphorylation levels were evaluated in the kidneys of UUO mice treated with either vehicle or DA. Consistent with the results from cultured fibroblasts, DA treatment significantly reduced β-catenin expression in UUO kidneys in a dose-dependent manner (Figure 5A). Moreover, DA restored pro-degradation phosphorylation of β-catenin at Ser33/37/45/Thr41, which had been diminished in UUO samples, supporting the hypothesis that DA facilitates β-catenin ubiquitin-mediated proteolysis in vivo (Figure 5B). Immunohistochemical analysis revealed that elevated β-catenin levels in fibrotic kidneys were predominantly localized to the tubules and interstitial regions, and this pathological increase was effectively reversed by DA treatment (Figure 5C). High-dose DA treatment restored β-catenin levels in UUO kidneys to values comparable to those of healthy mice.
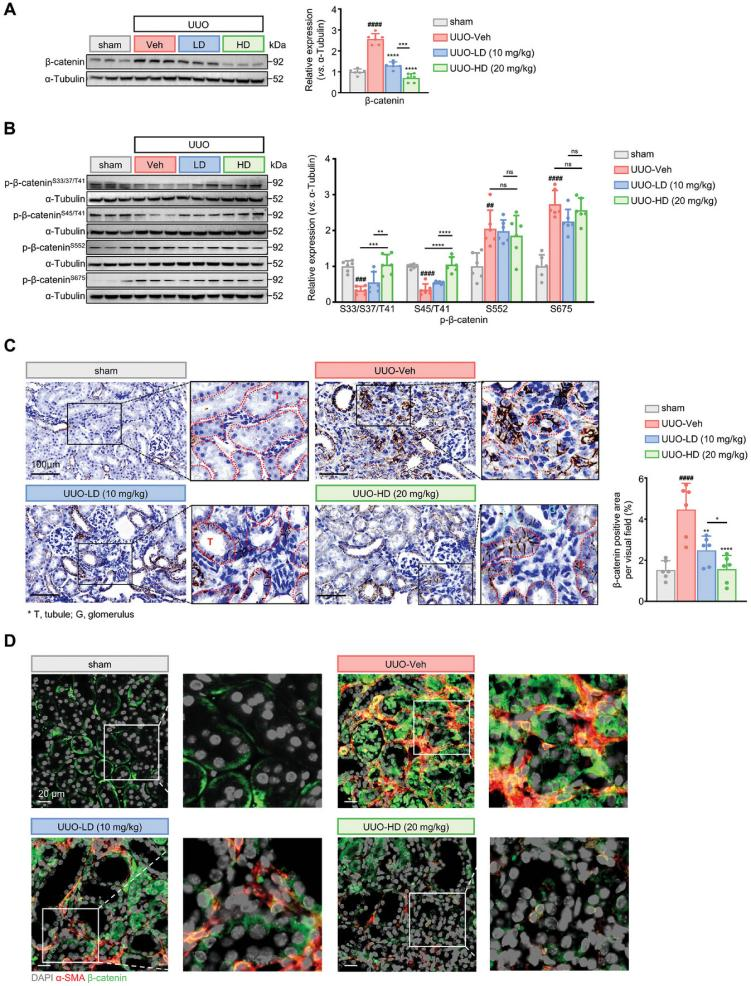
Figure 5. DA enhances p-β-catenin (S33/37/45/T41) levels while reducing total β-catenin expression in UUO kidneys.
6. DA Blocks Fibrosis-Driven β-Catenin Signaling by Targeting Cdc42 Activity
Thermal proteomics was employed to explore the mechanism by which DA inhibits β-catenin signaling in fibrosis. Cdc42 was identified as a potential target of DA, with findings emphasizing its essential role in cytoplasmic signal transduction. Validation through active Cdc42 pull-down assays, cellular thermal shift assays (CETSA), and surface plasmon resonance (SPR) experiments confirmed this interaction. Furthermore, molecular docking and residue mutation analyses pinpointed Leu119 and Lys128 as critical residues mediating DA-Cdc42 binding. These results shed light on the molecular basis of DA's pharmacological activity.
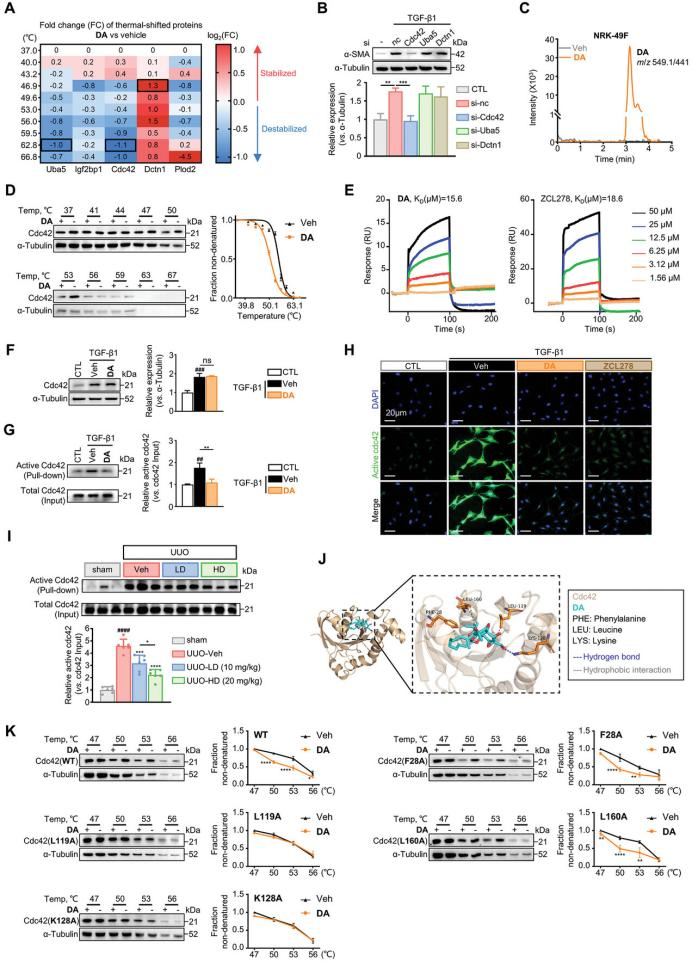
Figure 6. DA binds directly to Cdc42 and suppresses its activity.
7. DA Modulates the p-PKCζ/p-GSK-3β/β-Catenin Axis via Cdc42 to Exert Antifibrotic Effects
Functional studies demonstrated that Cdc42 silencing using small interfering RNA (siRNA) attenuated the antifibrotic effects of DA. In contrast, Cdc42 overexpression enhanced renal fibroblast activation and abolished DA's efficacy, confirming Cdc42 as the molecular target of DA. Mechanistically, Cdc42 deficiency promotes β-catenin degradation by reducing phosphorylation of protein kinase Cζ (p-PKCζ) and glycogen synthase kinase 3β (p-GSK-3β). Reduced p-GSK-3β corresponds to increased GSK-3β activity, which initiates β-catenin proteolysis through phosphorylation at Ser33/37/Thr41. Consistent with this, DA was shown to regulate renal fibroblast activation via the p-PKCζ/p-GSK-3β/β-catenin axis, as confirmed by experimental validation.
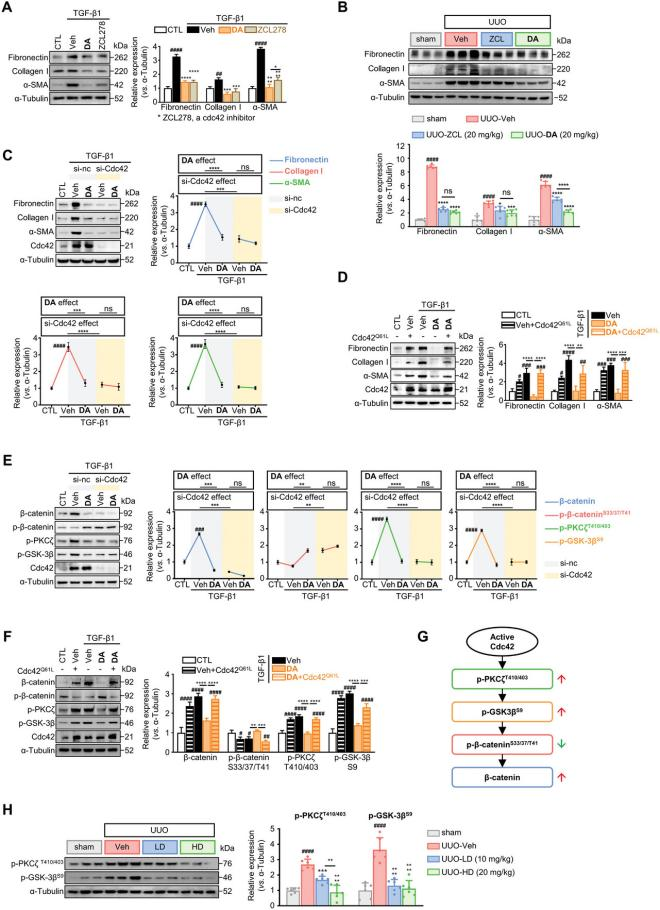
Figure 7. DA targets Cdc42 to activate p-PKCζ/p-GSK-3β-mediated phosphorylation of β-catenin at Ser33/37/Thr41.
8. Elevated Cdc42 Expression Is Linked to Renal Fibrosis in Mice and Humans
The clinical relevance of Cdc42 in CKD-associated renal fibrosis was examined using publicly available gene expression datasets. Analysis revealed that Cdc42 transcriptional levels were significantly higher in CKD patients compared to healthy controls (Figure 8B). Additionally, Cdc42 expression showed a strong positive correlation with key fibrotic markers, including Acta2, Col1a, Col3a1, and Fn1 (Figure 8C). Immunohistochemical analysis further demonstrated minimal Cdc42 expression in the tubules and glomeruli of healthy kidneys, with negligible presence in the interstitial regions. In contrast, kidneys from CKD patients exhibited markedly increased Cdc42 expression across tubules, glomeruli, and the interstitial space (Figure 8D), suggesting a potential role for Cdc42 in renal fibrosis progression. In summary, these findings highlight a robust association between elevated Cdc42 expression and renal fibrosis, indicating that targeting Cdc42 through pharmacological inhibition may offer therapeutic potential for CKD patients.
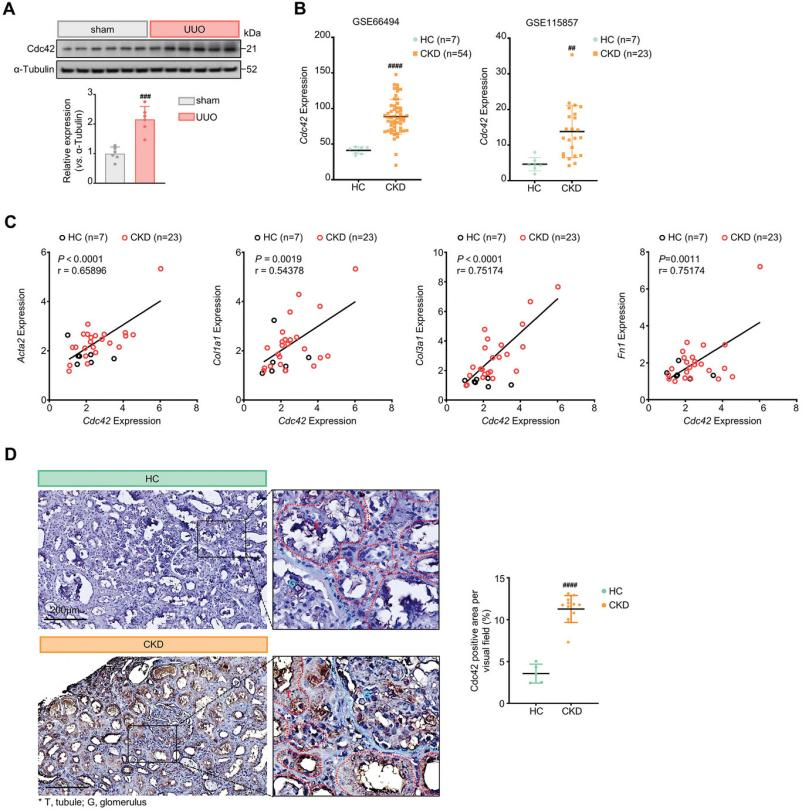
Figure 8. Cdc42 expression is significantly elevated in both UUO mice and CKD patients.
Research Conclusions
DA, a natural diterpenoid isolated from the medicinal plant W. chamaedaphne, is a potent antifibrotic agent. It effectively inhibits renal fibroblast activation and mitigates renal fibrosis in mouse models, demonstrating superior efficacy compared to the positive control, pirfenidone. Mechanistically, DA binds to Cdc42, suppressing its activity and subsequently downregulating downstream p-PKCζ and p-GSK-3β levels. This suppression enhances p-β-catenin phosphorylation at Ser33/37/Thr41, facilitating β-catenin degradation via the ubiquitin-proteasome pathway.
Future research and therapeutic strategies for renal fibrosis should prioritize the exploration of natural products and precise targeting of the Cdc42-mediated GSK-3β/β-catenin signaling pathway. Such approaches hold significant promise for advancing the prevention and treatment of chronic kidney disease (CKD). MtoZ Biolabs, as a provider of biological mass spectrometry services, specializes in high-resolution kidney tissue analysis to support the investigation of cellular and regulatory changes during renal fibrosis. We invite researchers to collaborate with us in elucidating the molecular mechanisms underlying renal fibrosis and developing targeted therapeutic strategies that address the global burden of kidney disease.
For detailed information on MtoZ Biolabs’ kidney tissue analysis services, please contact our technical support team.
References
[1] Hu X, Gan L, Tang Z, Lin R, Liang Z, Li F, Zhu C, Han X, Zheng R, Shen J, Yu J, Luo N, Peng W, Tan J, Li X, Fan J, Wen Q, Wang X, Li J, Zheng X, Liu Q, Guo J, Shi GP, Mao H, Chen W, Yin S, Zhou Y. A Natural Small Molecule Mitigates Kidney Fibrosis by Targeting Cdc42-mediated GSK-3β/β-catenin Signaling. Adv Sci (Weinh). 2024 Apr;11(13):e2307850. doi: 10.1002/advs.202307850. Epub 2024 Jan 19. PMID: 38240457; PMCID: PMC10987128.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
How to order?







