Quantum Yield Measurement Service
Quantum Yield Measurement Service is a quantitative characterization offering based on precision spectroscopy principles. It is designed to determine the efficiency with which a luminescent material or photosensitive system emits photons after excitation, reflecting its photoluminescence quantum efficiency.
Quantum Yield (QY) refers to the ratio of emitted photons to absorbed photons during photoexcitation. As a dimensionless value ranging from 0 (non-fluorescent) to 1 (100% efficiency), it quantifies the luminescent performance of a system. QY is a core parameter for evaluating photoluminescent materials, fluorescent probes, biological labels, and photosensitizers. It is widely used in materials science, optoelectronics, bioimaging, sensing, and drug development. QY directly affects key performance indicators such as fluorescence intensity, photostability, and detection sensitivity.
With the ongoing emergence of novel fluorescent materials—such as quantum dots, organic dyes, metal complexes, carbon dots, and upconversion nanoparticles—accurate QY measurement is essential for evaluating material quality, optimizing emission performance, comparing structural effects, and guiding application development.
MtoZ Biolabs offers Quantum Yield Measurement Service for solution-phase, solid-state, film, and nanocomposite samples. This service provides reliable data to support material optimization, performance evaluation, drug delivery, and bio-label development.
Technical Principles
The measurement of quantum yield is grounded in photophysical principles of energy conservation and fluorescence emission processes, and it typically follows these key principles:
1. Absorption and Emission Process
Upon excitation with a specific wavelength of light, electrons are promoted to an excited state.
They return to the ground state via radiative (fluorescent) or non-radiative (thermal dissipation, etc.) transitions.
Quantum yield represents the ratio of radiative transitions to the total number of excitation events.
2. Absolute Measurement (Integrating Sphere Method)
An integrating sphere collects emitted and unabsorbed excitation light in all directions uniformly.
The power of the excitation source, the intensity of residual excitation light, and the sample’s emitted fluorescence are recorded.
By integrating photon counts, QY is directly calculated as the ratio of emitted to absorbed photons—eliminating the need for reference standards.
3. Relative Measurement
The sample is measured under identical conditions alongside a reference dye with a known QY.
QY is calculated by comparing the integrated fluorescence intensity and absorbance of the sample and the standard.
Analysis Workflow
Quantum Yield Measurement Service at MtoZ Biolabs includes the following workflow:
1. Instrument Setup
Configure the integrating sphere system or cuvette module, and set excitation wavelengths and detection parameters.
2. Spectral Acquisition
Collect the excitation spectrum, absorption spectrum, and emission fluorescence spectrum to ensure data completeness and accuracy.
3. QY Calculation and Correction
Calculate the ratio of emitted to absorbed photons based on the principle of energy conservation, with corrections for self-absorption and scattering where applicable.
4. Report Output
Deliver a comprehensive technical report, including experimental conditions, raw spectral data, calculation process, and QY results.
Applications
Typical application scenarios for Quantum Yield Measurement Service include:
Characterization of Fluorescent Nanomaterials
Efficiency assessment of quantum dots, carbon dots, metal-organic frameworks (MOFs), and upconversion particles.
Evaluation of the effects of surface modification or shell coatings on emission properties.
Development of Biological Labels and Probes
Emission performance analysis of fluorescent dyes and proteins.
Fluorescence retention studies under varying pH, solvents, and temperature conditions
Material Development for Organic/Inorganic Optoelectronic Devices
QY testing of emissive components in OLEDs, LEDs, solar materials, and photosensitizers.
Efficiency comparison under different doping or hybridization strategies.
Optimization of Drug Delivery and Imaging Systems
Evaluation of imaging efficiency for fluorescently labeled drugs and nanocarriers.
Screening of signal output units with high signal-to-noise ratio and biocompatibility.
FAQ
Q. What is the fundamental difference between absolute and relative QY measurement methods, and how should one choose?
Absolute QY measurement uses an integrating sphere to directly collect absorbed and emitted light from the sample, offering high accuracy and reproducibility without relying on reference dyes. It is suitable for various sample forms, including solutions, powders, and thin films.
Relative QY measurement, by contrast, involves comparing the sample to a standard fluorophore with a known QY (e.g., quinine, rhodamine). While simpler and faster, it requires careful control of absorbance, solvent refractive index, and environmental conditions—making it more suitable for quick screening of solution-phase samples.
In general, absolute methods are recommended for high-precision needs, especially in comparative studies or materials/device development.
Q. How important is the choice of reference dye in relative QY measurement? Should the emission wavelengths be similar?
It is critically important. The reference dye should have an emission spectrum that closely matches that of the sample to ensure consistent detector response and spectral integration conditions. MtoZ Biolabs uses validated standards such as quinine sulfate (blue), rhodamine 6G (yellow-green), and fluorescein (green), carefully selected based on the sample’s spectral characteristics to avoid systematic errors.
Case Study
This study employed absolute quantum yield measurement using an integrating sphere setup to quantitatively evaluate the emission efficiency of rare-earth-doped core–shell nanocrystals in the short-wave infrared (SWIR) region. Results showed that heterogeneous shell structures (e.g., CaF₂) significantly suppressed cation intermixing at the core–shell interface, greatly enhancing luminescence. Quantum yield reached as high as 50%, notably higher than traditional homogeneous-shell structures (e.g., NaYF₄). These results directly validated the critical role of crystal structure design in controlling SWIR emission performance and provided quantitative evidence for developing high-efficiency bioimaging nanomaterials.
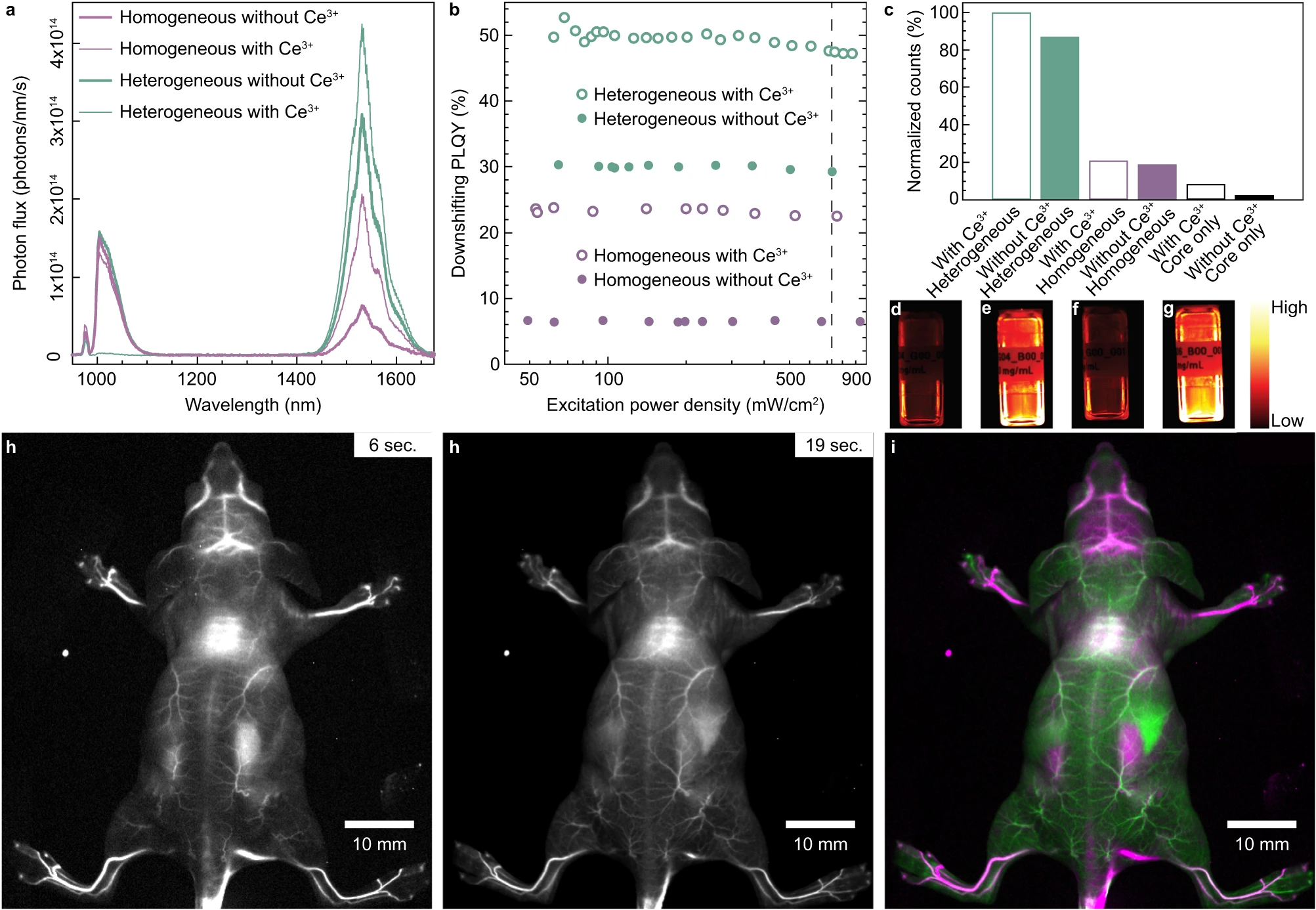
Cardona F A. et al. Nature Communications. 2023.
How to order?







