Antibody C-Terminal Lysine (K) Deletion Ratio Analysis Service
During antibody production, different levels of heterogeneity usually occur, potentially affecting antibodies' biological function. One of the primary sources of this heterogeneity is the deletion of lysine (K) at the C-terminus of the heavy chain, and it is a common phenomenon in recombinant antibodies produced by mammalian cells. As lysine is a positively charged basic amino acid, antibodies without this C-terminal lysine display a distinct charge state compared to those retaining it.
The loss of C-terminal lysine is attributed to the specific cleavage of C-terminal basic amino acids by intracellular carboxypeptidases. However, this enzymatic cleavage is not complete, resulting in the presence of different isoforms (K0, K1, K2) of lysine depletion within the antibody samples. The analysis of the ratio of K deletion can reflect the extent of lysine loss at the C-terminus of the antibody heavy chain.
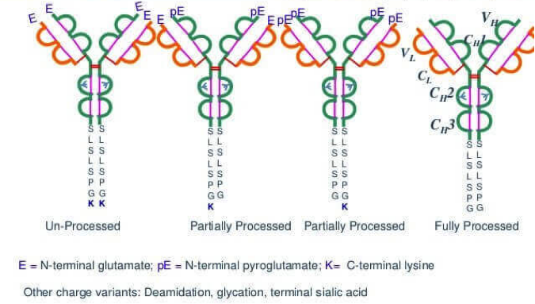
Figure 1. Monoclonal Antibodies with Different C-Terminal Lysine (K) Deletion Forms
(K0: no loss, K1: one missing, K2: two missing)
MtoZ Biolabs offers comprehensive detection services for antibody C-terminal K deletion, with our team capable of analyzing the deletion ratios for monoclonal antibodies from various species sources and subtypes.
Owing to lysine's inherent positive charge, its absence reduces the antibody's positive charge, enabling the differentiation of antibodies with and without C-terminal lysine deletion via charge-based separation methods. The loss of C-terminal lysine (K) alters the sequence of the peptide fragments at the antibody's C-terminus following enzymatic digestion. Quantitative results for these two types of C-terminal peptides (with or without K deletion) can reflect the extent of lysine depletion at the protein molecule's C-terminus. By using tandem mass spectrometry technology to achieve qualitative detection and quantification, the proportion of antibody C-terminal K deletions can be accurately analyzed.
Analysis Workflow
1. Sample Pre-Treatment
Sample pre-treatment involves centrifugal filtration, desalting, concentration measurement, and enzymatic digestion of antibodies.
2. Mass Spectrometry Data Collection
Peptide fragments are separated via liquid chromatography, and their mass-to-charge ratio information is acquired using high-precision mass spectrometry.
3. Data Analysis
Dedicated mass spectrometry software is utilized for the qualitative and quantitative analysis of K deletion antibody C-terminal peptides, determining the relative abundance and proportion of antibody C-terminal lysine isoforms.
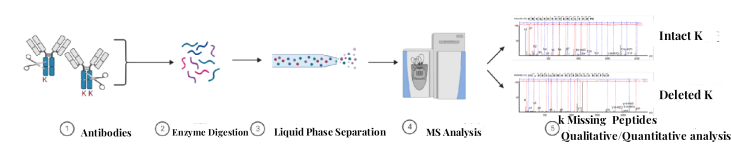
Figure 2. Detection Workflow for Antibody C-Terminal Lysine Deletion
Service Advantages
1. High-precision mass spectrometry instruments ensure accuracy and precision. The high-resolution Orbitrap Fusion™ Lumos™ Tribrid™ mass spectrometer significantly enhances detection accuracy.
2. Results reports are provided within two weeks.
3. Applicable to a wide range of monoclonal antibodies from various species.
Sample Results
1. Experimental Steps
2. Mass Spectrometry Parameters
3. Raw Data
4. Secondary Mass Spectrum of Antibody C-terminal K-deleted Peptide Fragments
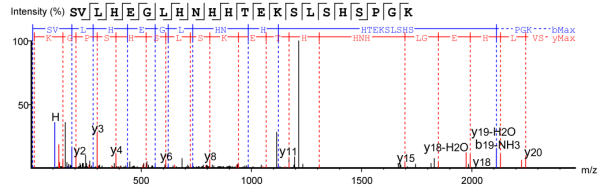
Figure 3. Peptide Fragment with Intact C-terminal K
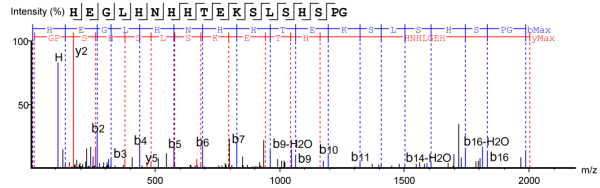
Figure 4. Peptide Fragment with Deleted C-terminal K
5. Table of Antibody C-terminal K Deletion Types and Ratios

Sample Submission Requirements
1. Submitted monoclonal antibody samples must exceed 100 micrograms.
2. The detectable types of C-terminal lysine (K) deletion in antibodies include K0, K1, and K2.
3. We have previously analyzed antibody types such as IgG, IgM, IgD, etc.
Services at MtoZ Biolabs
1. Identification of the Type of C-Terminal Lysine (K) Deletion in Antibodies
2. Quantification of the Proportion of C-Terminal Lysine (K) Deletion in Antibodies
Applications
1. Detection of Antibody Heterogeneity induced by C-Terminal Lysine Deletion
2. Quality Control for Antibody Therapeutics
FAQ
Q1: What are the impacts of C-terminal lysine deletion on antibodies?
The deletion of C-terminal lysine in antibodies can affect the antibody-mediated complement activation process.
Q2: What is the quantitative basis for analyzing the proportion of C-terminal lysine deletion in antibodies using mass spectrometry?
The quantitative basis involves the amino acid sequence of the antibody's C-terminal peptide fragments and their relative intensities. The proportion of C-terminal lysine deletion is calculated using the ratio of the quantitative results.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Antibody Characterization Service
How to order?







