Biopharmaceutical Process-Related Impurities Analysis Service
In biopharmaceutical engineering and bioproduct production, the detection and control of process-related impurities are the core of product quality management. These impurities may include cell matrix (host residual proteins, host residual DNA), cell cultures (inducers, antibiotics, or culture medium components), and by-products produced in downstream processes. These impurities can not only affect the purity and efficacy of the product but also, in some cases, may pose potential safety risks. Therefore, accurate information about process-related impurities is key to ensuring the quality and safety of bioproducts. High-performance liquid chromatography (HPLC), mass spectrometry (MS), nuclear magnetic resonance (NMR), and various electrophoresis techniques provide powerful tools for the analysis of process impurities. By combining these analytical techniques, qualitative and quantitative analysis of various impurities produced during the production process can be performed.
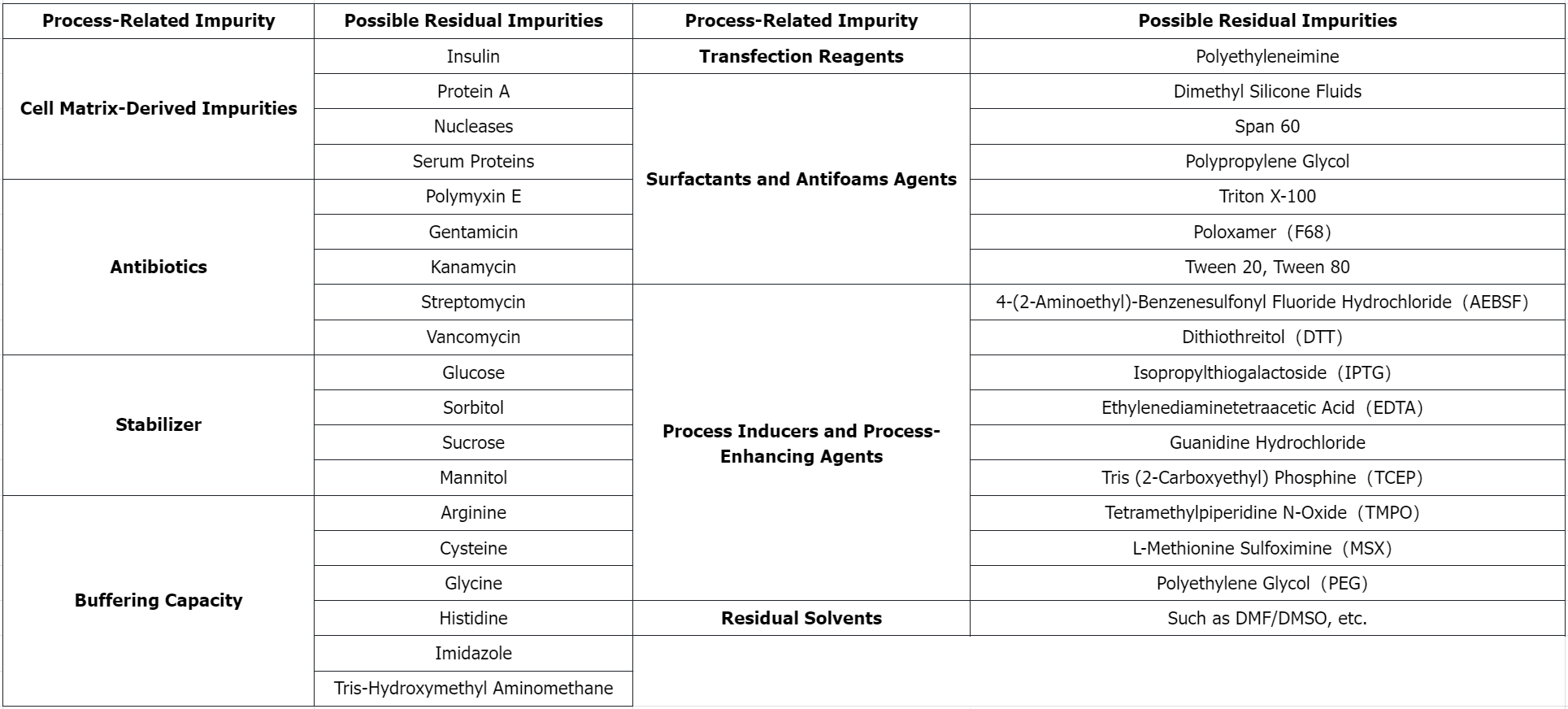
Figure 1. MtoZ Biolabs's Process-Related Impurity Detection List
MtoZ Biolabs uses its existing bioproduct physicochemical analysis platform and structural characterization platform to provide methods for the analysis of process-related impurities, offering comprehensive and accurate impurity analysis services, including basic qualitative analysis as well as in-depth quantitative analysis and risk assessment. MtoZ Biolabs has a team of experienced experts who can provide you with efficient, customized solutions to meet a variety of research needs, providing comprehensive support in bioproduct R&D and production. Free consultation is available to learn more details.
Deliverables
1. Experimental Procedures
2. Relevant Mass Spectrometry Parameters
3. Detailed Information on Process-related Impurity Analysis
4. Mass Spectrometry Images
5. Raw Data
How to order?







