Antibody Sequencing Service: Advanced NGS and MS Techniques
Antibodies (Abs) are essential components of the adaptive immune system, which can combat infections and other harmful agents. Produced by B cells, Ab is either secreted or expressed on the plasma membrane at the present of B cell receptors (BCRs). Each Ab comprises two heavy chains (VH) and two light chains (VL). The VL each include a constant region varing only across different classes and subclasses of immunoglobulin (Ig), and a variable region characterized by a sequence of highly variable amino acids and responsible for antigen recognition.
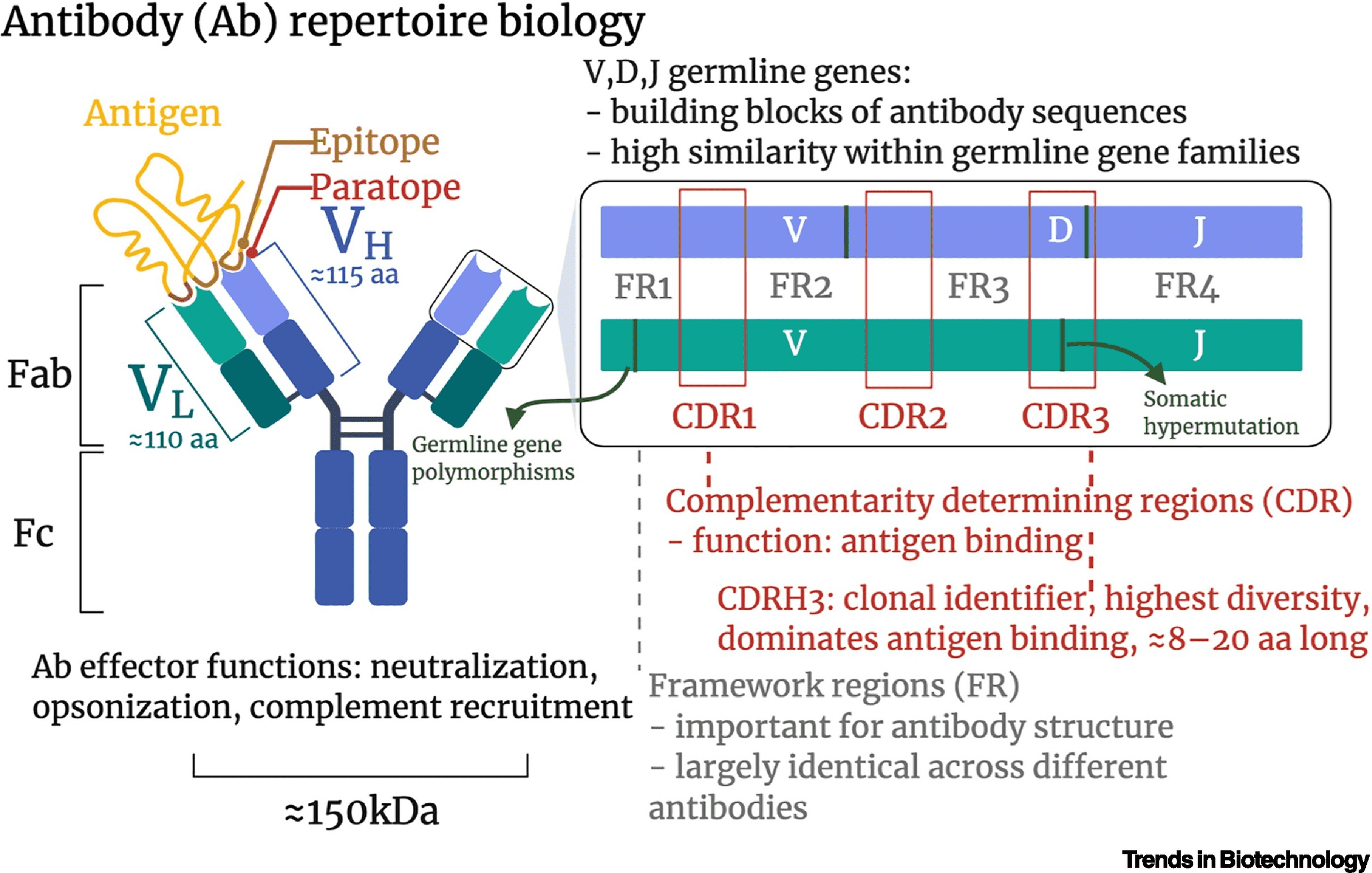
Figure 1. Structure of Antibody [1]
The variability of the variable region primarily promotes the vastness and adaptability of human antibody repertoire that is essential for neutralizing antigens. The antibody repertoire is extensive with 1016-1018 unique antibody sequences. Pro-B cells undergo V(D)J recombination, which diversifies Ig. The variable (V), diversity (D), and joining (J) gene segments come together to form the variable region exon of an Ig, initially expressed as IgM in unmatured B cells. When antigen invades in oragnism, B cells are activated, leading to further diversification of Ig through somatic hypermutation (SHM) and class switch recombination (CSR).
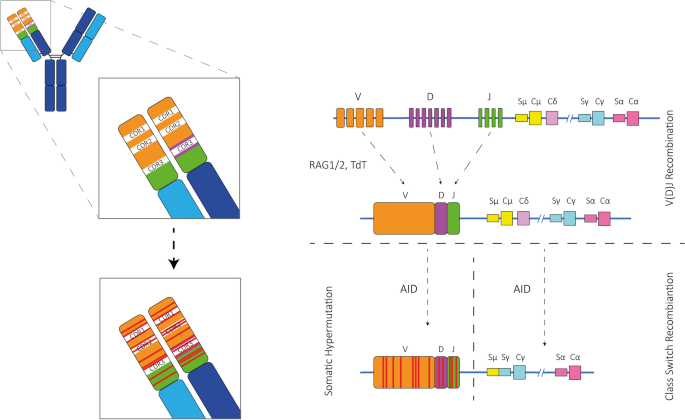
Figure 2. Antibody Diversification Mechanisms [2]
Abs have rapidly been deployed in clinical therapies, emerging as one of the fastest-growing categories of new drugs. Therapeutic antibodies are typically discovered through hybridoma and display methods. The hybridoma method involves creating perpetuated hybridoma cell lines that produce specific Abs by fusing myeloma cells with spleen cells from immunized animals (usually rodents) with a target soluble antigen. Despite its success, the hybridoma method relys on sequence of Ab of rodent animals, which may elicit immunogenic responses, necessitating humanization the sequence of Ab of rodent animals to reduce its immunogenicity. Display techniques in vitro connect the genetic information of antibodies (genotype) with their antigen-binding capabilities (phenotype). Various display systems, including phage display system and yeast display system, have been developed. Abs can be applied in diagnosis, imaging, and therapy, holding substantial clinical value. Although protein-level antibody libraries in serum and mucosa contribute significantly to humoral protection, the unexplored sequence composition and dynamics of these libraries limit comprehensive understanding of critical immunological and biotechnological parameters.
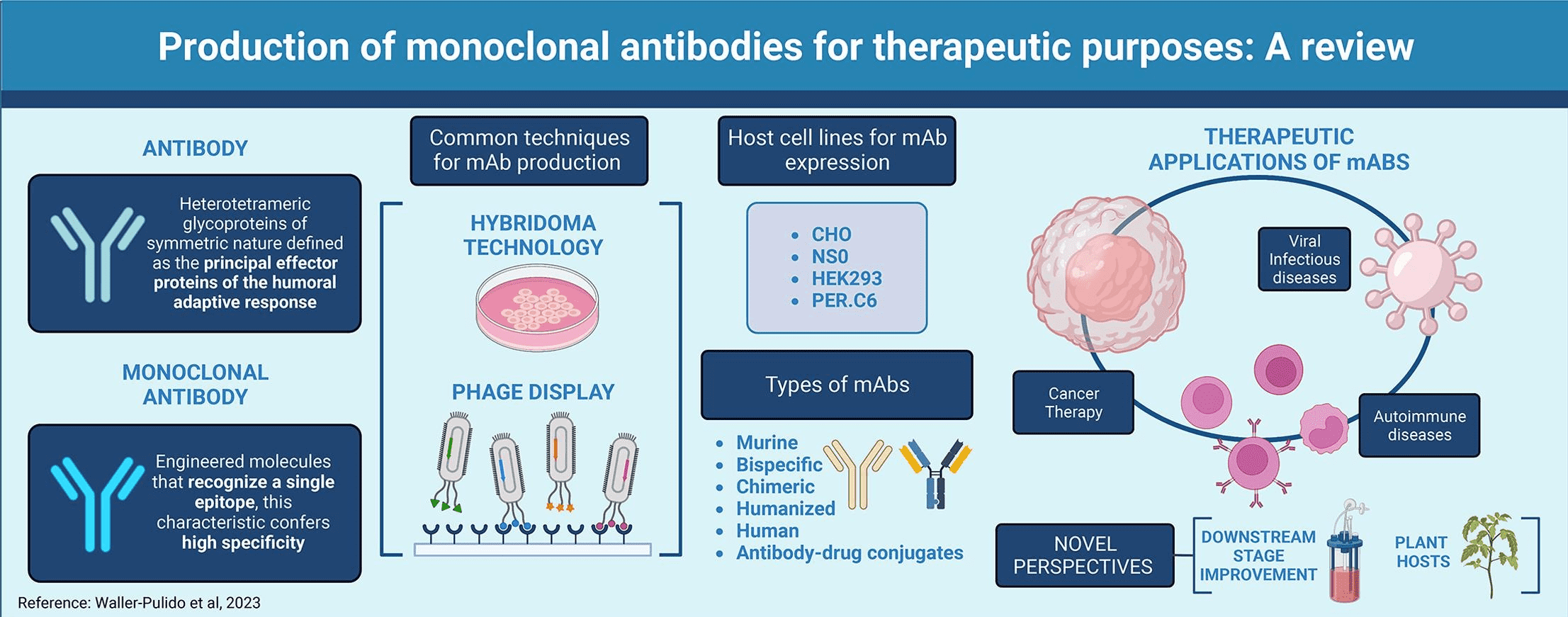
Figure 3. Production and Applications of Antibodies [3]
MtoZ Biolabs eatablishs a comprehensive platform for antibody sequencing analysis with complete data infoemation, retrieval, and analysis. The platform provides a range of services, including the production of various mAbs (such as those from rabbits and mice), antibody sequencing (gene sequencing and de novo protein sequencing), Ab reactivity screening, single B-cell sorting and sequencing, and large-scale expression of recombinant Abs. These services support extensive research like animal immunization, antibody sequencing, screening and production. Our team employs a range of experimental gradients to identify optimal conditions to decode antibody gene and protein structures by collecting data from sequencers and mass spectrometers and analysing through bioinformatics. MtoZ Biolabs offers one-stop services from animal immunization to antibody production, effectively addressing antibody research and development.
References
[1] Snapkov I, Chernigovskaya M, Sinitcyn P, Lê Quý K, Nyman TA, Greiff V. Progress and challenges in mass spectrometry-based analysis of antibody repertoires. Trends Biotechnol. 2022 Apr;40(4):463-481. doi: 10.1016/j.tibtech.2021.08.006. Epub 2021 Sep 14. PMID: 34535228.
[2] de Brito PM, Saruga A, Cardoso M, Goncalves J. Methods and cell-based strategies to produce antibody libraries: current state. Appl Microbiol Biotechnol. 2021 Oct;105(19):7215-7224. doi: 10.1007/s00253-021-11570-x. Epub 2021 Sep 15. PMID: 34524471.
[3] Alejandra WP, Miriam Irene JP, Fabio Antonio GS, Patricia RR, Elizabeth TA, Aleman-Aguilar JP, Rebeca GV. Production of monoclonal antibodies for therapeutic purposes: A review. Int Immunopharmacol. 2023 Jul;120:110376. doi: 10.1016/j.intimp.2023.110376. Epub 2023 May 25. PMID: 37244118.
How to order?







