Bacterial Contraction Injection System Service
One of the main obstacles in drug development is how to deliver drugs effectively to target cells. In general, macromolecules such as proteins and mRNA cannot penetrate the cell membrane because the cell membrane has acquired protective mechanisms through evolution. However, viruses can deliver their genetic material into cells. Therefore, viruses and virus-like particles are widely used to deliver DNA, mRNA, and proteins for therapeutic purposes.
Micro-organisms have developed efficient biological mechanisms to break through host membrane barriers via endocytosis, membrane fusion, or pore penetration. Therefore, bacteria have also been modified as gene therapy vectors and even used in clinical trials. However, successful cases have been limited to inactivated or attenuated vaccines, possibly due to strong immune responses and low transfer efficiency. Many bacterial pathogens can use specialized protein secretion systems to secrete virulence factors from their cell membrane into host cells or the host environment, making them attractive candidate delivery vectors. Although previous studies have shown that endosymbiotic bacteria can deliver proteins to mouse cells, it is still unclear whether this system can be reconstructed to target human cells, which is a key step in drug development.
At present, a programmable protein delivery vector based on the extracellular contractile injection system (eCIS) has been developed. These modified syringe-like macromolecules can inject protein cargo of interest into eukaryotic cells (including human cells) in a targeted manner, showing potential applications in gene therapy, cancer treatment, and biocontrol. Secreting proteins that modulate host biology is a common strategy for bacteria to enhance their capabilities. eCISs are a family of such molecular injectors, resembling the tail of bacteriophages, and can specifically bind to target cell on the baseplates via tail fibers. After target cell recognition, sheath contraction is triggered, forcing spike proteins and payload proteins through the cell membrane into the inner lumen to complete the delivery process.
eCIS can be easily reprogrammed to carry protein cargo of interest and target specific cells, providing additional protein delivery tool for various research and application fields.
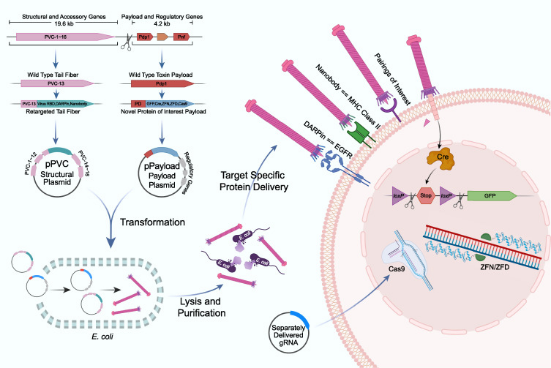
Figure 1. Engineering, Production and Targeted Injection of eCIS [1]
MtoZ Biolabs provides a comprehensive bacterial contractile injection system construction platform. The platform offers services including the construction of recombinant bacterial CISs, in vitro production of photorhabdus virulence cassette (PVC), quality control of PVC, and functional validation at the cellular and animal levels, enabling research in CISs and related fields. Our team can explore optimal experimental conditions through multi-gradient experiments and design targeted PVCs to investigate the applications of bacterial contractile injection systems across various biological fields. MtoZ Biolabs offers vector design, sample preparation, quality control, and multi-level experimental verification services, providing one-stop solutions to bacterial contractile injection system-related issues.
On March 29, 2023, Zhang Feng's research team at MIT published their findings on programmable protein delivery by bacterial contractile injection systems in Nature. They demonstrated that an eCIS from the insect pathogen Photorhabdus asymbiotica, known as PVC, targetd specific receptors through the distal binding elements of its tail fibers. Utilizing computational structure-guided tail fiber engineering, they showed that PVC can be reprogrammed to target organisms that these systems did not naturally, including human and mice cells, with nearly 100% efficiency. Finally, they demonstrated that PVC can be loaded with various protein payloads, including Cas9, base editors, and toxins, and functionally delivered them into human cells. These results showed that PVCs were programmable protein delivery devices with potential applications in gene therapy, cancer treatment, and biocontrol.
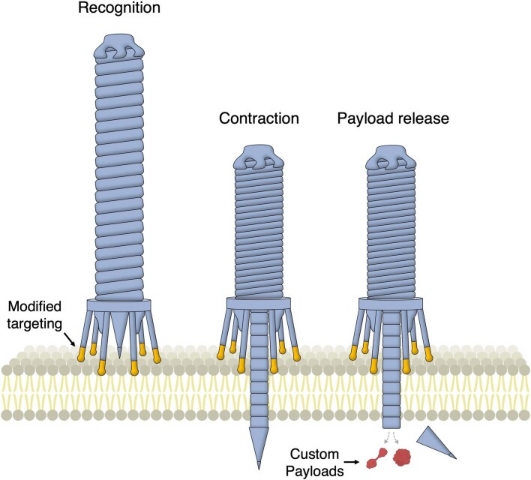
Figure 2. Programmable Protein Delivery Using Bacterial Contraction Injection System [2]
In endosymbiotic bacteria, the secretion of host-modulating factors typically benefits the adaptability of symbionts. This process is mediated by several complex systems that can actively deliver protein payloads into cells. One example is the contractile injection system (CIS), a syringe-like nanomachine resembling bacteriophage tails. The CIS is a macromolecular complex containing a rigid tube structure located within a contractile sheath. The contractile sheath is anchored to the baseplate and sharpened by spike proteins. The payload is believed to be loaded into the inner tube lumen behind the spike, either as a fusion protein with the spike tube or bound to the spike itself. After target cell recognition, the spike is forced through the membrane via sheath contraction.
CIS can anchor to bacterial membranes to form contact-dependent delivery systems known as Type VI secretion systems (T6SS), or attach to the thylakoid membranes in cyanobacteria (tCIS), where they are activated during cellular stressed responses. They can also be produced as free eCIS and released extracellularly to deliver payloads independently of their bacterial producers. eCISs are widely distributed among bacteria and archaea and have been classified into at least six families.
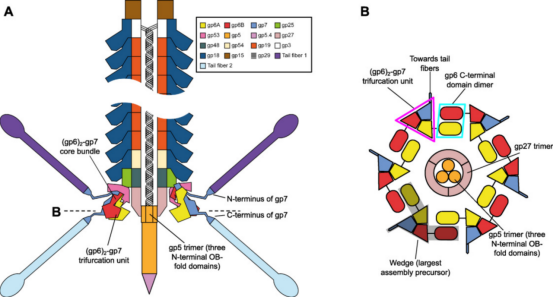
Figure 3. Organizational Structure of CIS [3]
Although the structure of the CIS is complex, it can be significantly simplified when viewing phage tails, R-type pyocins, T6SS, Serratia antifeeding prophages (Afp) and others as verticallu stacked repeats of tail tube protein hexamers surrounded by sheath protein hexamers. This structure is composed of baseplates and capping assemblies at both ends. The baseplate coordinates host recognition or other environmental signals with sheath contraction, while the capping assembly prevents the tube from sliding out of the sheath during contraction. In phage T4 tails, except for the terminal repeats consist of the same pair of proteins: gene product (gp) 19 forms the tube, and gp18 forms the sheath. The baseplate-proximal end of the tube consists of gp48 and gp54 hexamers, while the distal end consists of gp3 hexamers. Gp3 interacts with the capping protein gp15, terminating the sheath and the tube. The hexamers formed by gp15 are larger than the tube hexamers but much smaller than the sheath disks.
Certain structural features of T4 baseplate and their relative conservation across other CIS enable us to infer a possible evolutionary pathway for baseplates from simpler assemblies. The key components of the conserved baseplate region are the (gp6)2-gp7 heterotrimer. The structural domains of gp6 and gp7, which form this heterotrimer, are the same, suggesting a common ancestor with a gp6-like fold. The ancestral gp6-like protein includes a trimerization module (composed of a core bundle and trifurcation unit) and a dimerization module (which doubles as a host-interacting or sensing domain). The N-terminus region of this protein could form a homotrimeric core bundle, while the C-terminal domain could dimerize and bind to certain structures on the bacterial cell surface. The emergence of separate genes encoding gp7-like proteins specializes in receptor-binding functions, thereby enhancing binding affinity, while gp6-like proteins specializes in baseplate circularization. Finally, the duplication of the gp7 gene's receptor-binding domain generates a bona fide tail fiber gene, allowing fine-tuning and acceleration of the evolutionary and adaptation processes, leading to more specific recognition of target cells.
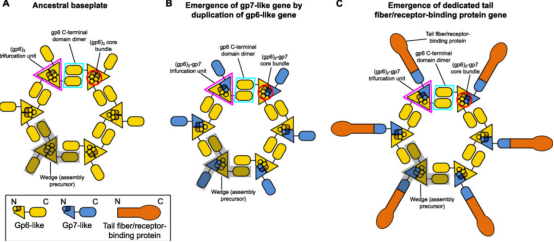
Figure 4. Evolutionary Pathways of CIS [3]
Taking programmable protein delivery with a bacterial contractile injection system as an example, this paper introduces the research process of the system:
(1) Reconstruction and Engineering Design of eCIS
This research focused on a subtype of eCIS known as PVCs. PVCs are eCISs produced by members of the genus Photorhabdus, existing as endosymbionts in insect entomopathogenic nematodes. PVCs consisted of an operon of approximately 20 kb, containing 16 core genes (pvc1-16), which were essential for assembling a functional injection system. Downstream of pvc1-16 were the payload Pdp1 and Pnf. Like all eCISs, it was believed that these payload proteins entered target cells through the contraction of the PVC sheath and subsequent disassembly of the spike complex.
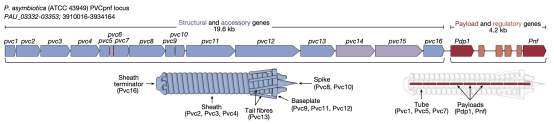
Figure 5. Structure of PVCs [2]
The study first employed Escherichia coli to produce PVCs from P. asymbiotica ATCC 43949 (PVCpnf). To facilitate downstream manipulation, the PVC system was divided into a separate structural plasmid and accessory plasmid (pPVC) and a payload and regulatory plasmid (pPayload).
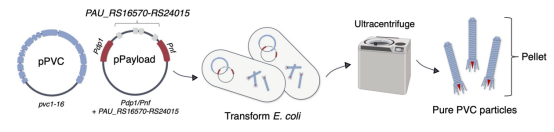
Figure 6. Production Process of PVCs [2]
The resulting protein complexes were analyzed using negative-stain transmission electron microscopy (TEM). The structures resembled canonical eCISs, containing intact baseplates and sheath structures approximately 116 nm in length.
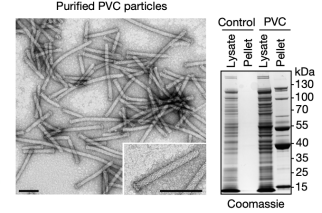
Figure 7. Electron Microscopy Structure of PVCs [2]
pPayload was essential for producing detectable PVC particles. This indicated that the small genes in the payload region were crucial for PVC formation in E. coli. When these purified complexes were briefly exposed to cultured Sf9 insect cells (selected because they were related to the insect endogenously targeted by PVCpnf24), they bound tightly to the cell surface. These results suggested that E. coli can be used to produce PVC complexes that were both proper assembly and targeting.
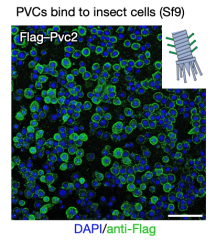
Figure 8. In Vitro Produced PVCs Can Target Cells [2]
To develop PVC sinto programmable protein delivery devices, the researchers next attempted to load novel non-native payload proteins into PVCs. In the presence of pvc15 (an ATPase also shown to be essential for loading payloads), all three novel payloads co-purified with PVCs, confirming that N-terminal fusion of the packaging domain was a general strategy for loading novel proteins into PVC particles.
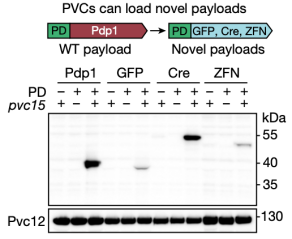
Figure 9. Non-Native Payload Proteins Can Be Loaded into PVC Particles [2]
The study then tested whether PVC-mediated protein delivery (with endogenous and engineered payloads) could be directly observed in cultured insect cells. After incubating Sf9 cells with unmodified PVCs harbouring native toxin payloads, researchers observed robust cytotoxicity. Moreover, when administered to Sf9 cells harbouring a Cre reporter system (loxP-GFP) were injected, PVCs artificially loaded Cre produced GFP signals, indicating that novel protein payloads can be functionally delivered via PVC. These results demonstrated that recombinant PVCs were biologically active against cultured insect cells and can be reprogrammed to load and deliver non-native proteins target cells to yield novel biological activities.
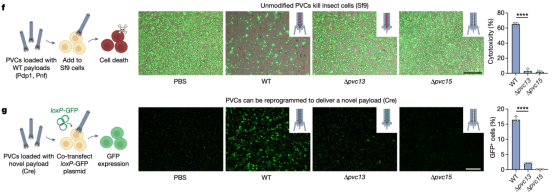
Figure 10. PVC Can Deliver Unnatural Payload Proteins to Target Cells and Kill Them [2]
(2) Changing the Tropism of PVC towards Human Cells
The mechanism by which PVC bound to target cells remains unclear. Based on the structure of bacteriophages, researchers hypothesized that the tail fiber, encoded by the pvc13, was responsible for recognition. To verify this, researchers designed two different binding domains: one derived from the trimeric knob domain from human adenovirus 5 (Ad5), and the other being the epidermal growth factor receptor (EGFR)-specific designed ankyrin repeat protein (DARPin) E01. The chosen contents were SpCas9, commonly used in CRISPR, an gene-editing component zinc finger deaminase (ZFD), and the native biotoxins Pdp1/Pnf. In A549 human lung adenocarcinoma cells (they overexpressed EGFR and were sensitive to Ad5 infection, which can be targeted by EGFR-specific DARPin), the efficiency of modified PVC was remarkably high, with minimal misidentification of non-target cells, resulting in successful gene editing and cell killing. The results suggested that Pvc13 was the tropism-determining element of the PVC and that this protein can be modified to produce predictable changes in target specificity.
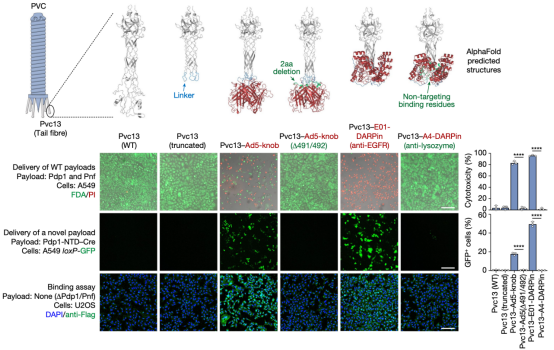
To further explore the potential of PVC as a protein delivery tool, researchers expanded their investigation by establishing several useful delivery applications in human cells. The first test involved reprogramming PVC to load and deliver Streptococcus pyogenes Cas9 (SpCas9) for gene editing in human cells. The results showed that when Pvc13-Ad5-knob redirected PVC was loaded with Cas9, the resulting particles induced on-target insertions and deletions (indels) in HEK 293FT cells containing guide RNA. Cas9 was much larger than the payloads natively loaded by this PVC (170 kDa for Pdp1-NTD-Cas9 compared to 37 kDa for Pdp1 and Pnf), indicating that PVC can deliver diverse payloads of various sizes.
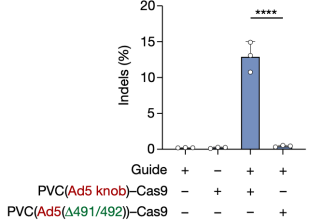
Figure 12. PVC Can Deliver Payloads of Different Sizes [2]
To achieve RNA-free gene editing with PVCs, researchers next attempted to deliver ZFDs. When PVCs retargeted with Pvc13–Ad5-knob were loaded with either the left or right arm of a ZFD targeting the human TRAC locus (ZFD-L or ZFD-R, respectively) and were co-administered to HEK 293FT cells, researchers observed on-target G-to-A base substitution, indicating that PVCs can deliver ZFDs to effect base editing in human cells
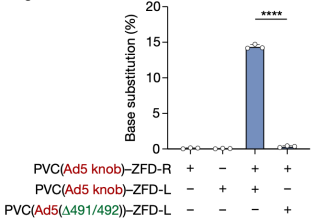
Figure 13. PVC Can Achieve Base Editing in Human Cells [2]
Finally, inspired by the endogenous biological function of PVCs (targeted killing via toxin delivery), researchers tested whether PVCs could be used for the specific killing of human cancer cells. They found that PVCs loaded with endogenous toxins (Pdp1 and Pnf) produced highly efficient cytotoxicity in Jurkat cells when retargeted with a DARPin specific for a T cell receptor. Notably, PVC targeting the myeloid receptor not produced by Jurkat cells (CD11b) resulted in negligible cell death, indicating that PVC-mediated cytotoxicity in human cancer cells was receptor-specific.
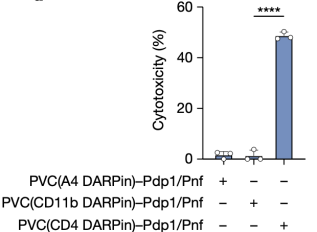
Figure 14. PVC Can Be Targeted in the Cytotoxicity of Human Cells [2]
(3) Assessing PVC Target Specificity
To investigate the target specificity of PVC, researchers firstly developed a panel of artificial HEK 293FT-derived cell types expressing defined non-native receptors that could easily be targeted by engineered PVCs.
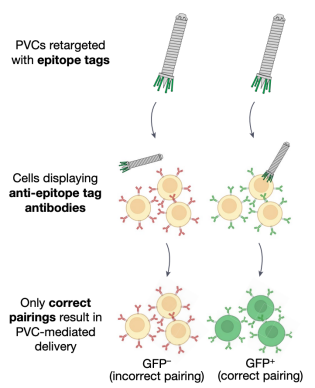
Figure 15. PVC Targeted Cell Construction [2]
For simplicity, researchers selected a panel of antibodies (scFvs and nanobodies) specific for commercial epitope tags as receptors. The associated panel of epitope tags were inserted into the distal binding domain of the tail fiber, and the modified PVCs were introduced into these "cell types" to observe how effectively PVCs could undergo target selection. It was found that PVCs retargeted with epitope tags could only efficiently deliver their payload to cells that specifically bound to these epitope tags. This result indicated that PVC specificity was primarily determined by the interaction between the tail fiber and its target receptor, and this interaction can be engineered to achieve specific recognition of novel cell types.
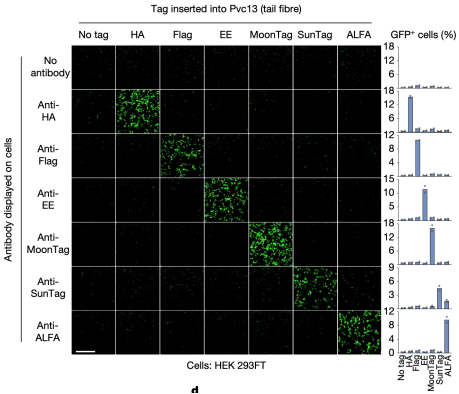
Figure 16. PVCs Specific Recognition of Target Cells [2]
Next, the researchers evaluated PVC specificity against EGFR, an natural receptor found on some human cell types. In this experiment, they tested whether PVCs programmed to target EGFR could specifically target cells known to express EGFR. It was found that PVCs retargeted with anti-EGFR DARPin (E01) and loaded with toxins (Pdp1 and Pnf) could only effectively kill EGFR-positive cell lines (A549 and A431) but not EGFR-negative cell lines (Jurkat and 3T3).
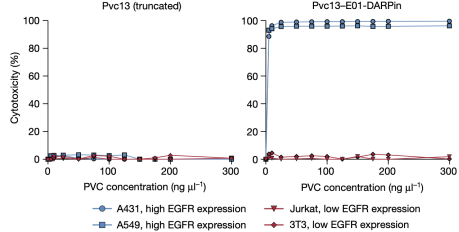
Figure 17. PVCs Specific Recognition of Target Cells [2]
(4) In Vivo Protein Delivery Using PVC
To determine whether PVC could ultimately be used in humans, researchers next attempted to deliver proteins in live mice. AlphaFold-guided engineering of Pvc13 was used to produce PVC variants capable of targeting mouse cells. Two new binding domains were screened: (1) a modified Ad5 knob domain (Ad5-knob(RGD/PK7)), previously used to expand the host range of Ad5 to mouse tissues, and (2) a nanobody targeting the mouse receptor (MHC class II). After incorporating these new binding domains into Pvc13, the resulting PVCs showed significantly enhanced activity in mouse cell lines and primary cells.
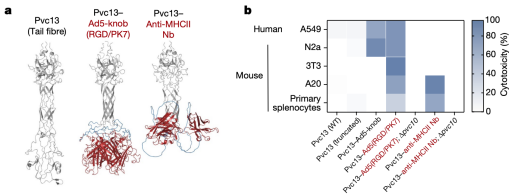
Figure 18. Modification of PVCs Variants [2]
After identifying a novel PVC design capable of targeting mouse cells, researchers next attempted to achieve protein delivery in vivo. Cre was loaded into PVCs containing Pvc13-Ad5-knob(RGD/PK7), and intracranial injection was performed in loxP-tdTomato reporter mice. Separately, PVC analogs lacking a spike tip (Pvc10) were also injected, revealing that this protein was essential for PVC-mediated delivery in vitro. Following intracranial injection of Pvc13–Ad5-knob(RGD/PK7) particles, Cre-mediated tdTomato expression was observed in the hippocampus, indicating that PVCs are active in vivo.
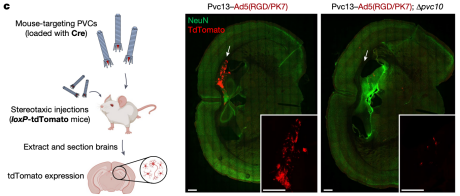
Figure 19. Protein Delivery by PVCs in Vivo [2]
Moreover, the mice did not exhibit any immune cell activation, inflammatory cytokine production, or weight loss. Seven days post-injection, PVCs were undetectable in the mouse brain, demonstrating the safety, efficacy, and rapid metabolism of this method.
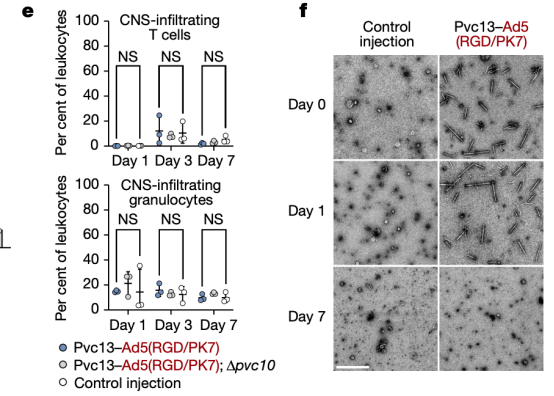
Figure 20. In Vivo Protein Delivery by PVCs Is Safe [2]
Analysis Workflow
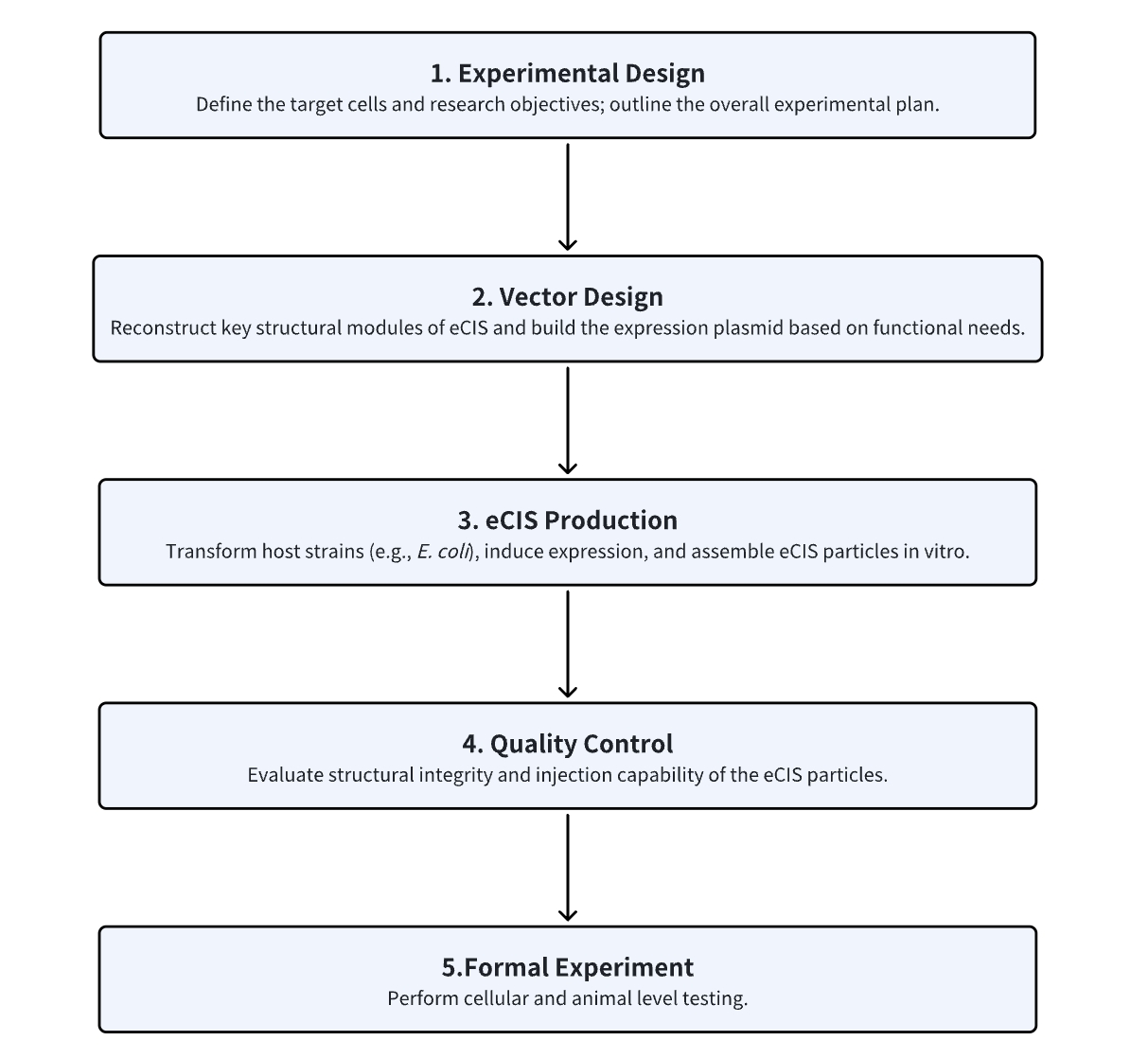
1. Determine the Experimental Procedure Based on Experimental Requirements
2. Design Recombinant eCIS
3. In Vitro Production of eCIS in E. coli
4. Assess eCIS Integrity and Target Specificity
5. Perform Cellular and Animal Experiments
Service Advantages
1. Design eCIS Based on Customer Requirements
2. Provide In Vitro eCIS Expression and Quality Control Services
3. Offer Cellular and Animal-Level Testing Services
Sample Results
1. A Bacterial Phage Tail-like Structures Kills Eukaryotic Cells by Delivering a Nuclease Effectors
Many bacteria interact with target organisms using syringe-like structures known as CIS. CIS is structurally similar to headness bacteriophages and shares evolutionarily related proteins, such as tail tubes, sheaths, and baseplate complexes. In many cases, CISs mediate trans-kingdom interactions between bacteria and eukaryotes by delivering effectors to target cells. However, the specific effectors and their modes of action remain largely unknown. To address this, researchers developed an in vitro model to study eCIS that targeted eukaryotic cells, called metamor-phosis-associated contractile structure (MACs). MACs killed two eukaryotic cell lines, the armyworm Sf9 cells and J774A.1 murine macrophage cells, by delivering the effector Pne1. Before the identification of Pne1, no CIS effectors with nuclease activity against eukaryotic cells had been described. These results revealed a novel mechanism of CIS-mediated bacteria-eukaryote interactions and represented a step towards developing CISs as novel delivery systems for eukaryotic hosts.
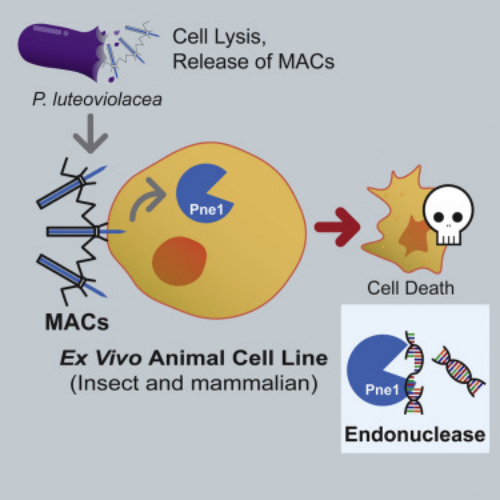
Figure 21. Bacteriophage Tails Kill Eukaryotic Cells by Injection of Nuclease Effectors [4]
2. Contraction Injection Systems Stimulate Tubeworms via Protein Effector Delivery
Many marine animals' swimming larvae decide where to settle on the seafloor based on the presence of specific bacteria. Despite the importance of microbe-animal interactions in the life cycles of various marine organisms, the specific biochemical cues that bacteria provide to induce these interactions have remained elusive. Tubeworm Hydroides elegans larvae are induced to settle by arrays of CISs resembling phage tail-like tails, which are released by the bacterium Pseudoalteromonas luteoviolacea. Researchers identified a new effector protein, Mif1, as responsible for this induction. Cryo-electron tomography imaging and functional assays showed that Mif1 acted as the cargo within the CIS tube lumen, and the mif1 gene was required for inducing metamorphosis. Purified Mif1 was sufficient for triggering metamorphosis when electroporated into tubeworm larvae. These findings suggested that the delivery of protein effectors by CISs may orchestrate microbe-animal interactions in diverse contexts.
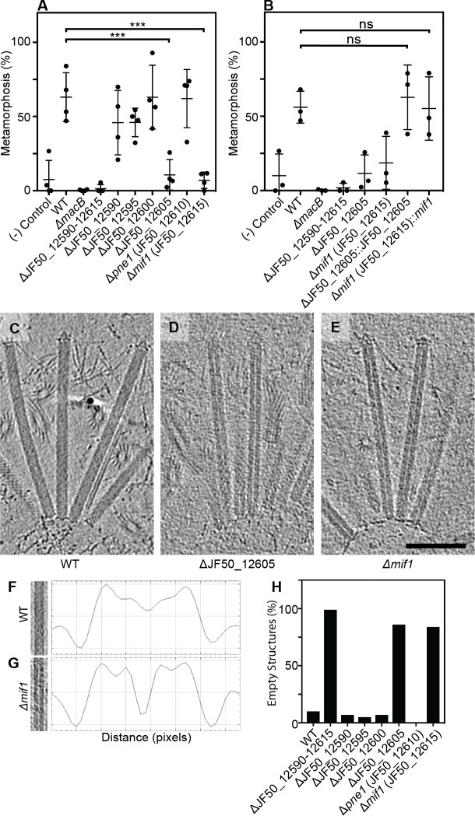
Figure 22. The CIS Stimulates Tube Worms by Translocating Protein Effectors [5]
3. N-Terminal Signal Peptides Enable PVC Complexes to Function as Efficient Protein Delivery Systems
eCISs are widely used bacterial nanomachines that resemble T4 phage tails. As a typical eCIS, PVC is proposed to inject toxins into eukaryotic cells by piercing the cell membrane from outside. This makes it an ideal tool for protein delivery in biomedical research. However, the manipulation of this nanocomplex as a molecular syringe is still not fully understood. A study identified a set of N-terminal signal peptide (SP) sequences crucial for loading effectors into the inner tube of the PVC complex. Using genetic operation, cryo-electron microscopy, in vitro translocation assays, and animal experiments, researchers found that under the guidance of SPs, many prokaryotic and eukaryotic proteins can be loaded into PVCs to exert their functions across cell membranes. Therefore, PVCs can be customized as potent protein delivery nanoinjectors for biotherapeutics by selecting a broad range of cargo proteins, regardless of their type, size, or charge.
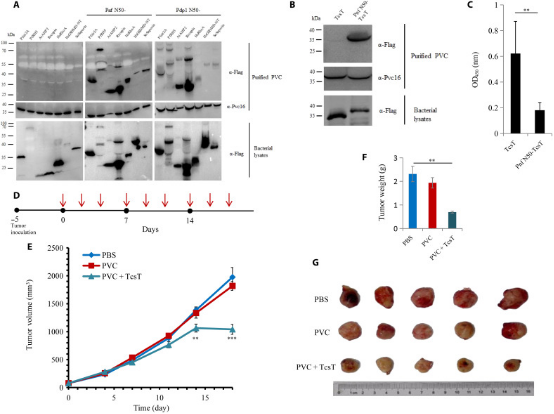
Figure 23. Anti-Tumor Applications of Eukaryotic Protein Loaded into PVC and in Mouse Models [6]
Sample Submission Requirements
1. Minimize Impurity Contamination
Services at MtoZ Biolabs
1. Complete Experimental Procedure
2. Relevant Instrument Parameters
3. Raw Experimental Data
4. Data Report
Applications
1. Cytoplasmic Contraction Injection Systems Mediate Streptomyces Cell Death
A study found that cytoplasmic CISs mediate cell death in Streptomyces bacteria. CISs are bacteriophage tail-like structures that mediate cell-cell interactions. Although CISs are abundant across various bacterial phyla, representative gene clusters in Gram-positive organisms remain understudied. A study characterized the CIS in the Gram-positive multicellular model organism Streptomyces coelicolor and demonstrated that, unlike most other CISs, the S. coelicolor CIS (CISSc) mediated cell death in response to stress and affects cellular development. CISSc was expressed in the cytoplasm of vegetative hyphae and was not released into the culture medium. The cryo-electron microscopy structure enabled the engineering of non-contractile and fluorescently labeled CISSc assemblies. Cryo-electron tomography revealed that CISSc contraction was associated with reduced cellular integrity. Fluorescence light microscopy further showed that functional CISSc mediates cell death in response to various types of stress. The loss of functional CISSc impaired hyphal differentiation and secondary metabolic production. Finally, the researchers identified three putative effector proteins, which when absent, phenocopied other CISSc mutants. These findings offered new functional insights into the CISs of Gram-positive bacteria and provided a framework for studying novel intracellular functions, including regulated cell death and life cycle processes in multicellular bacteria.
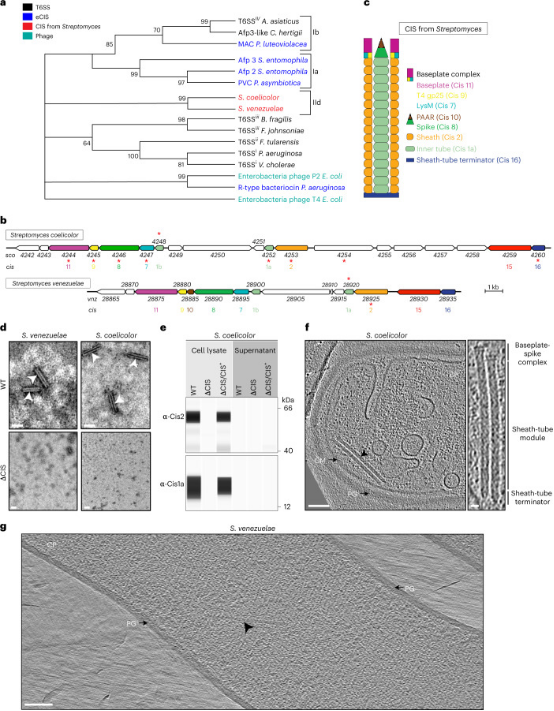
Figure 24. Cytoplasmic Contraction Injection System Mediates Streptomyces Cell Death [7]
References
[1] Lu S, Cai Y. Bacterial molecular syringe for drug delivery. Cell Host Microbe. 2023 Jun 14;31(6):917-919. doi: 10.1016/j.chom.2023.05.005. PMID: 37321174.
[2] Kreitz J, Friedrich MJ, Guru A, Lash B, Saito M, Macrae RK, Zhang F. Programmable protein delivery with a bacterial contractile injection system. Nature. 2023 Apr;616(7956):357-364. doi: 10.1038/s41586-023-05870-7. Epub 2023 Mar 29. PMID: 36991127; PMCID: PMC10097599.
[3] Taylor NMI, van Raaij MJ, Leiman PG. Contractile injection systems of bacteriophages and related systems. Mol Microbiol. 2018 Apr;108(1):6-15. doi: 10.1111/mmi.13921. Epub 2018 Feb 26. PMID: 29405518.
[4] Rocchi I, Ericson CF, Malter KE, Zargar S, Eisenstein F, Pilhofer M, Beyhan S, Shikuma NJ. A Bacterial Phage Tail-like Structure Kills Eukaryotic Cells by Injecting a Nuclease Effector. Cell Rep. 2019 Jul 9;28(2):295-301.e4. doi: 10.1016/j.celrep.2019.06.019. PMID: 31291567.
[5] Ericson CF, Eisenstein F, Medeiros JM, Malter KE, Cavalcanti GS, Zeller RW, Newman DK, Pilhofer M, Shikuma NJ. A contractile injection system stimulates tubeworm metamorphosis by translocating a proteinaceous effector. Elife. 2019 Sep 17;8:e46845. doi: 10.7554/eLife.46845. PMID: 31526475; PMCID: PMC6748791.
[6] Jiang F, Shen J, Cheng J, Wang X, Yang J, Li N, Gao N, Jin Q. N-terminal signal peptides facilitate the engineering of PVC complex as a potent protein delivery system. Sci Adv. 2022 Apr 29;8(17):eabm2343. doi: 10.1126/sciadv.abm2343. Epub 2022 Apr 29. PMID: 35486720; PMCID: PMC9054023.
[7] Casu B, Sallmen JW, Schlimpert S, Pilhofer M. Cytoplasmic contractile injection systems mediate cell death in Streptomyces. Nat Microbiol. 2023 Apr;8(4):711-726. doi: 10.1038/s41564-023-01341-x. Epub 2023 Mar 9. PMID: 36894633; PMCID: PMC10066040.
How to order?







