cGMP Analysis Service
- Qualitative analysis of cGMP metabolites
- Quantitative analysis of cGMP content
- Provide scientific foundation for development and improvement
- Animal tissue: ≥ 100 mg
- Plant tissue: ≥ 200 mg
- Serum/ Plasma: ≥ 200 μL
- Urine: ≥ 2 mL
cGMP (cyclic guanosine monophosphate) is a cyclic nucleotide formed by a guanine base, ribose, and a phosphate group linked by a phosphodiester bond. Its phosphate group forms a cyclic structure through an ester bond with the 3' and 5' hydroxyl groups of the ribose. cGMP is an important intracellular second messenger that plays a key role in various physiological processes and signaling pathways. It is primarily synthesized from GTP (guanosine triphosphate) by the action of guanylate cyclase (GC) and is inactivated by phosphodiesterases (PDEs) that degrade it to 5'-GMP. cGMP plays a crucial role in regulating vascular smooth muscle relaxation, neural signal transmission, cell proliferation, and apoptosis.
The cGMP signaling pathway is a target for various drugs. For example, PDE5 inhibitors are used to treat erectile dysfunction and pulmonary arterial hypertension, while sGC activators are used to treat heart failure and hypertension. Drugs that regulate cGMP levels also show potential applications in the treatment of cardiovascular diseases, neurodegenerative diseases, and certain cancers. Changes in the levels of cGMP and its related metabolites can serve as biomarkers for certain diseases. For instance, elevated cGMP levels in cardiovascular diseases may be associated with endothelial dysfunction and impaired vasodilation. Analyzing changes in cGMP levels can be used for early diagnosis and monitoring of disease progression and treatment efficacy.
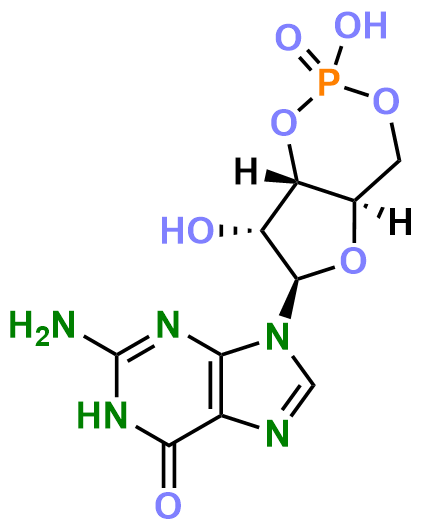
Figure 1. The Structure of cGMP
MtoZ Biolabs is proud to present our state-of-the-art cGMP Analysis Service. Utilizing our cutting-edge liquid chromatography-mass spectrometry (LC-MS) platform and a team of seasoned metabolomics experts, our service ensures accurate and sensitive analysis of cGMP.
Analysis Workflow
Service Advantages
1. Continuously refined protocols and cutting-edge analytical tools
2. Expert experimental planning
3. Rapid processing times
4. Exceptional precision, selectivity, and sensitivity
Applications
Sample Submission Requirements
1. Sample Types
We accept serum, plasma, urine, tissue, or other biological samples. A minimum of 3 samples under identical conditions is required.
2. Sample Volume
3. Sample Preservation
Samples must be stored at -80°C to ensure stability.
Note: Please provide precise details about sample collection and handling.
Deliverables
1. Experimental Procedures
2. Relevant Liquid Chromatography and Mass Spectrometry Parameters
3. Detailed Information on cGMP
4. Raw Data
5. Custom Analysis Report
Our cGMP analysis service is designed to support your research and product development endeavors, by providing dependable and precise results.
How to order?







