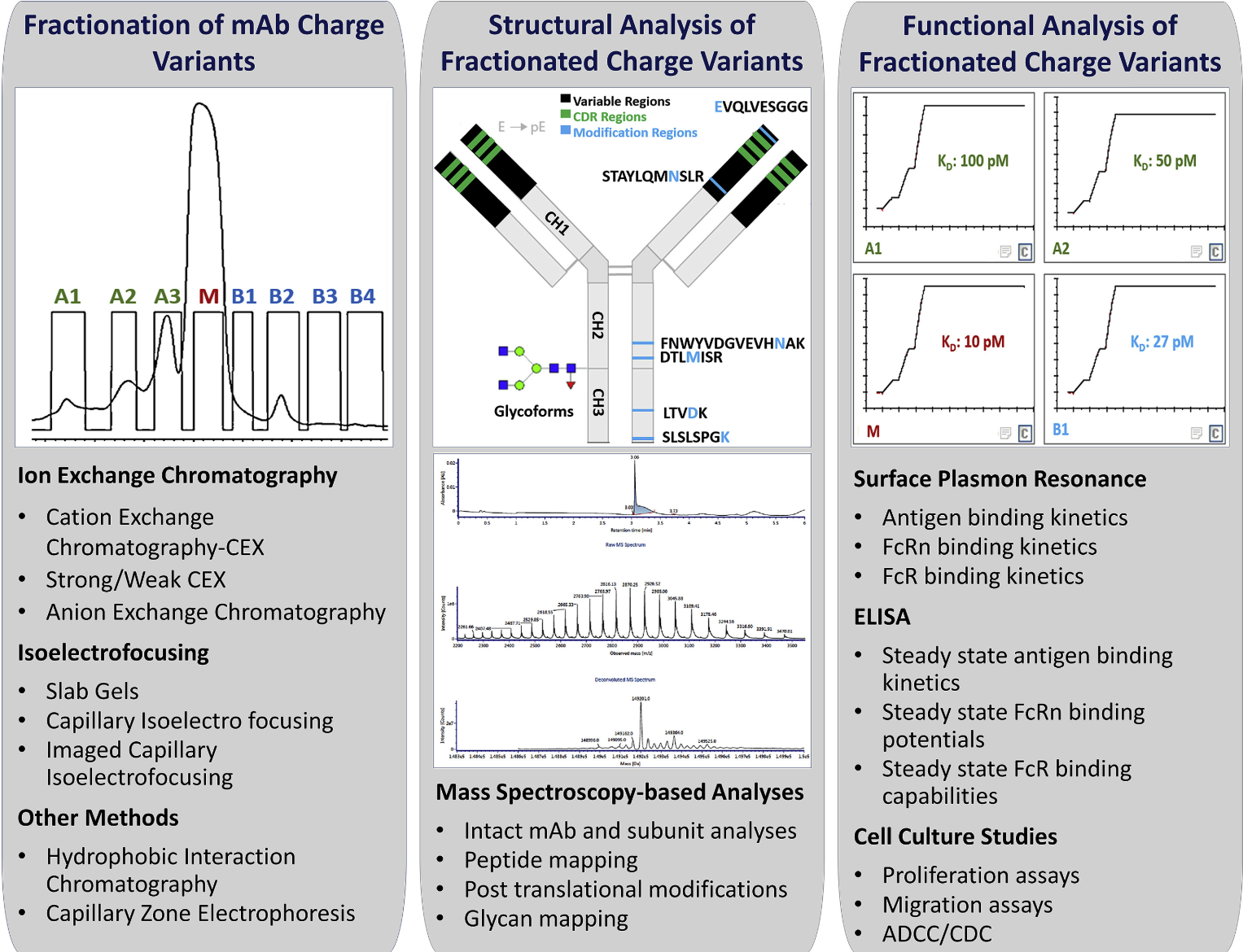
Charge Variant Analysis Service
Charge variant analysis is a powerful technique for identifying and characterizing the heterogeneous charge states of proteins that arise from post-translational or chemical modifications, such as deamidation, oxidation, or glycosylation. In the context of protein therapeutics, these modifications often shift a protein’s isoelectric point (pI), resulting in charge variants that can significantly impact therapeutic efficacy, stability, and immunogenicity. As the biopharmaceutical market continues to grow, manufacturers must ensure consistent production quality and meet the stringent requirements set forth by regulatory authorities. Charge variant analysis has become crucial for mitigating risks associated with compromised performance or unwanted immune responses, especially in high-stakes applications such as monoclonal antibodies (mAbs).
In essence, charge variant analysis reveals how these modifications affect protein folding, stability, and biological activity.Beyond its fundamental role in elucidating protein structure and behavior, charge variant analysis serves multiple applications across the biopharmaceutical lifecycle. During early-phase research, it helps identify potential liability sites on a therapeutic protein, enabling researchers to optimize sequence designs or production conditions. In later stages, it informs formulation and process development by highlighting conditions—such as pH or buffer composition—that minimize unwanted modifications and maintain product integrity. Additionally, charge monitoring is indispensable for lot-to-lot consistency, offering the means to detect low-level variants that could compromise patient safety or reduce clinical efficacy. As regulatory bodies tighten quality expectations, having a robust charge variant profiling strategy not only de-risks development but also expedites product approvals.
Service at MtoZ Biolabs
To address these challenges comprehensively, MtoZ Biolabs offers a fully integrated Charge Variant Analysis Service tailored to diverse protein therapeutics, including monoclonal antibodies, fusion proteins, and novel biologic platforms. Leveraging both classical methods (ion-exchange chromatography, capillary electrophoresis) and advanced technologies (mass spectrometry), our expert team provides end-to-end support—from method development and sample preparation to data interpretation and regulatory documentation. We work closely with clients to customize protocols that pinpoint critical quality attributes, ensuring consistent production and reduced risk of immunogenic responses. Whether you are in early discovery or nearing product licensure, our Charge Variant Analysis Service delivers high-resolution insights that streamline decision-making and enhance product profiles.
Service Advantages
1. Advanced Analysis Platform: MtoZ Biolabs established an advanced Charge Variant Analysis Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
2. One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
3. High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrates all Charge Variant Analysis Service data, providing clients with a comprehensive data report.
4. Fast Turnaround: The process from sample handling to report generation is efficient, shortening the analysis cycle.
5. Customizable Service: Personalized service to meet various research needs.
Case Study
Subunit-Level Charge Variant Analysis of Stressed Trastuzumab
Trastuzumab is known for its inherent charge heterogeneity, which intensifies under physiological stress (pH 7.4, 37 °C). To overcome the challenge of isolating pure variants at the intact level, a subunit-focused charge variant analysis approach was adopted using pH-gradient cation-exchange chromatography following selective enzymatic cleavage with GingisKHAN®. In the figure, Fab fragments (A4, A3, A2, A1, M, B1, B2, B3) are baseline-separated, each bearing distinct modifications illustrated by colored dots. Subsequent LC–MS/MS peptide mapping and MALDI-ISD FT-ICR MS identified three key alterations: deamidation at Asn-30 in the light chain (red), isomerization at Asp-102 in the heavy chain (blue), and pyroglutamate formation at the heavy chain’s N-terminus (green). This subunit-based charge variant analysis strategy provides clearer insights into multi-site modifications, facilitating enhanced monitoring of antibody stability and quality during biopharmaceutical development.
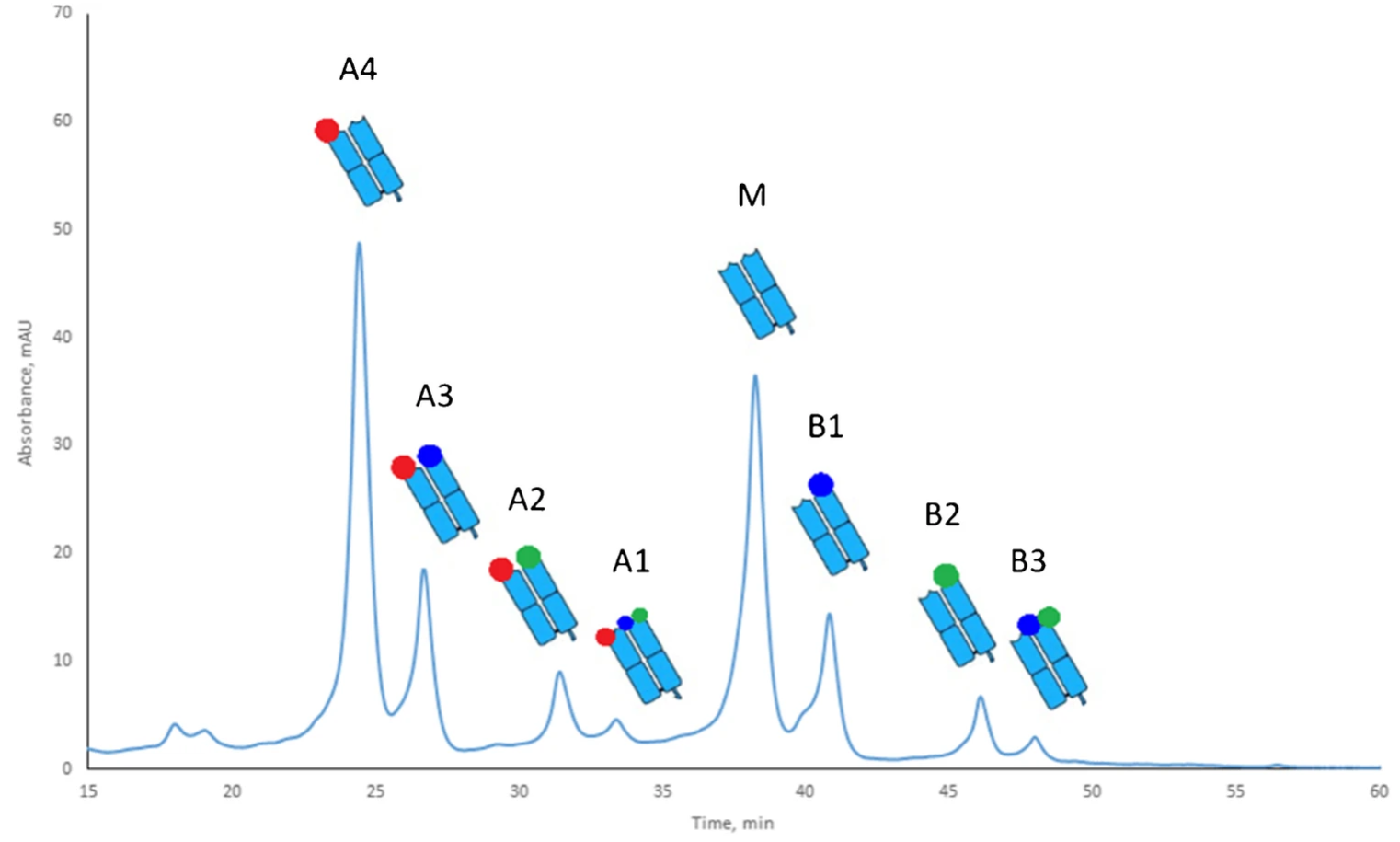
Spanov, B. et al. Anal Bioanal Chem. 2023.
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Total Ion Chromatogram & Quality Control Assessment
4. Data Analysis, Preprocessing, and Estimation
5. Bioinformatics Analysis
6. Raw Data Files
For more information about our Charge Variant Analysis Service, please contact us.
How to order?







