Crosslinking Protein Interaction Analysis Service
Under physiological conditions, most protein interactions are transient, posing significant challenges for their study. Crosslinking reagents resolve this by covalently bonding interacting proteins, thereby capturing protein-protein complexes and preserving brief, weak interactions for easier separation and characterization. Researchers can perform crosslinking both in vivo and in vitro, choosing based on project needs. In vivo crosslinking maintains proteins in their native states, while in vitro crosslinking might lead to protein denaturation.
1. In Vivo Crosslinking
In vivo, crosslinking reactions take place within cells, reducing false positives and maintaining complex stability. Hydrophobic and lipophilic crosslinkers are used for proteins inside the cell membrane, whereas hydrophilic and water-soluble crosslinkers are preferred for membrane-surface proteins like plasma-anchored receptors. Despite proteins remaining in their native states, the complexity within cells makes optimization challenging. However, using crosslinkers with shorter arms can help mitigate non-specific crosslinking.
2. In Vitro Crosslinking
This method involves homogenizing and lysing cells, making it ideal for studying stable interactions. Lysing cells in specific buffers facilitates controlled reaction conditions such as temperature, pH, and ionic strength. The absence of in vivo environmental constraints allows for a broader selection of crosslinkers and better control of specificity.
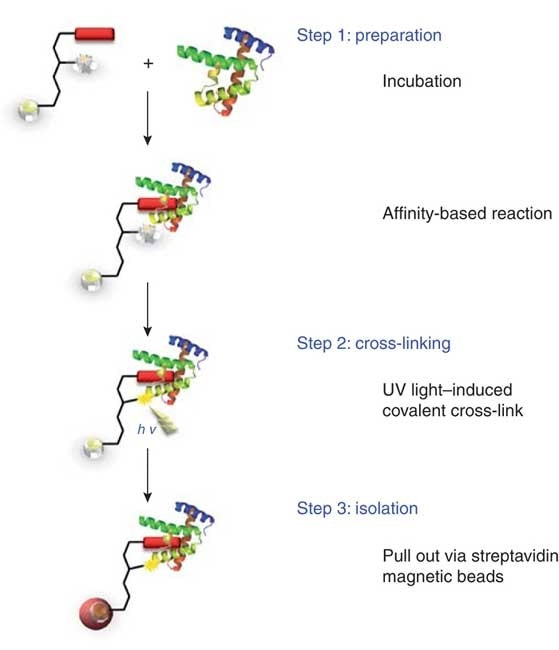
Dülsner, E. et al. Nat Methods. 2009.
Figure 1. Analysis of Protein Interaction Using Cross-Linking Method
Common Types
1. Chemical Crosslinking
Chemical crosslinkers covalently connect interacting proteins, structural domains, or peptides that are closely adjacent due to interactions, by forming chemical bonds between specific amino acid functional groups. Depending on the specific requirements of the project, many commercial chemical crosslinkers are available, including homobifunctional or heterobifunctional crosslinkers, long-arm or short-arm crosslinkers, cleavable or non-cleavable crosslinkers, water-soluble or non-water-soluble crosslinkers, etc. Homobifunctional molecules target the same functional groups on proteins, while heterobifunctional crosslinkers can target different functional groups on different proteins, allowing for greater variability or specificity in crosslinking. Crosslinker molecules can also be designed to include cleavable elements, such as esters or disulfide bonds, which can be reversed or disrupted by adding hydroxylamine or a reducing agent, respectively. Hydrophobic crosslinkers can enter hydrophobic protein structural domains or pass through cell membranes; Hydrophilic crosslinkers can interact well with the aqueous environment and promote the formation of crosslinked structures.
2. Photosensitive Crosslinking
To better analyze protein-protein interactions, more complex crosslinkers have been designed by adding photosensitive groups, etc. These photosensitive groups only react at selected times and only respond to ultraviolet light exposure. Heterobifunctional crosslinkers with a chemical crosslinking group on one end and a photosensitive group on the other can react with the selected target protein in two steps.
Service Advantages
1. Analysis of Transient in Vivo Protein Interactions
2. Analysis of Low-Affinity in Vitro Protein Interactions
3. High Detection Sensitivity
Analysis Workflow
1. Design of Crosslinking Methods and Types of Crosslinkers Based on Specific Project Requirements: In Vivo/In Vitro
2. Optimization of Reaction Conditions, Targeting a Combination of Sample Characteristics and Project Requirements, for Experimental Condition Optimization
3. Cell Lysis and Co-Immunoprecipitation (Co-IP)
4. Immunoblotting/Mass Spectrometry Analysis (as Determined by Project Requirements)
5. Analysis of Data and Delivery of Report
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
MS-Based Protein-Protein Interaction Analysis Service
How to order?







