Cryo-Electron Microscopy Based Service
Structural biology is a field grounded in molecular biology, biochemistry, and biophysics, focusing on the detailed study of biomacromolecules such as proteins and nucleic acids. Its goal is to elucidate the fine structural details of biomacromolecules to understand the mechanisms behind their functional roles, thereby enhancing our understanding of life's processes. Furthermore, as many therapeutic agents target proteins, structural biology significantly influences drug development, offering clear insights from basic research to pharmaceutical applications.
The primary techniques for determining the three-dimensional (3D) structures of proteins include X-ray crystallography, nuclear magnetic resonance (NMR), and cryogenic electron microscopy (cryo-EM). X-ray crystallography, which offers the highest resolution, requires the formation of protein crystals. This method faces challenges with large molecules and membrane proteins due to difficulties in crystallization. NMR spectroscopy is suited for analyzing small molecular weight proteins (typically under 20 kDa) and their interaction sites. In contrast, cryo-EM is ideal for studying large molecular weight protein complexes (often well over 100 kDa) and subcellular structures, making it necessary to integrate these methods for comprehensive structural analysis.
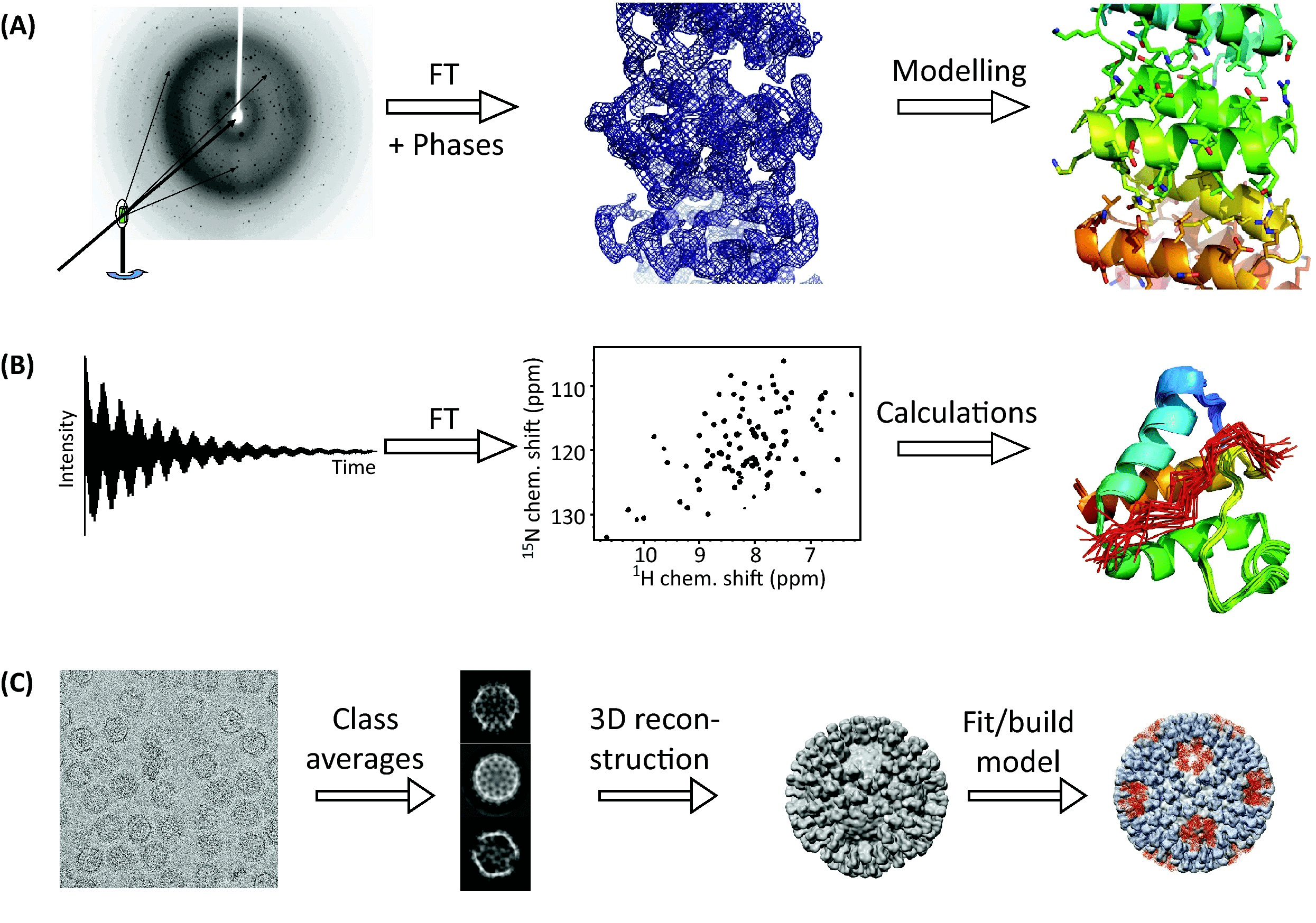
Figure 1. The Three Principal Techniques of Structural Biology [1]
Cryo-EM is a technique that uses a transmission electron microscope to observe specimens at low temperatures. It has become a crucial tool in structural biology, particularly through methods like single-particle analysis (SPA) and cryogenic electron tomography (cryo-ET). Other techniques, such as microcrystal electron diffraction (MicroED) and cryogenic scanning transmission electron microscopy (cryo-STEM), have also become popular.
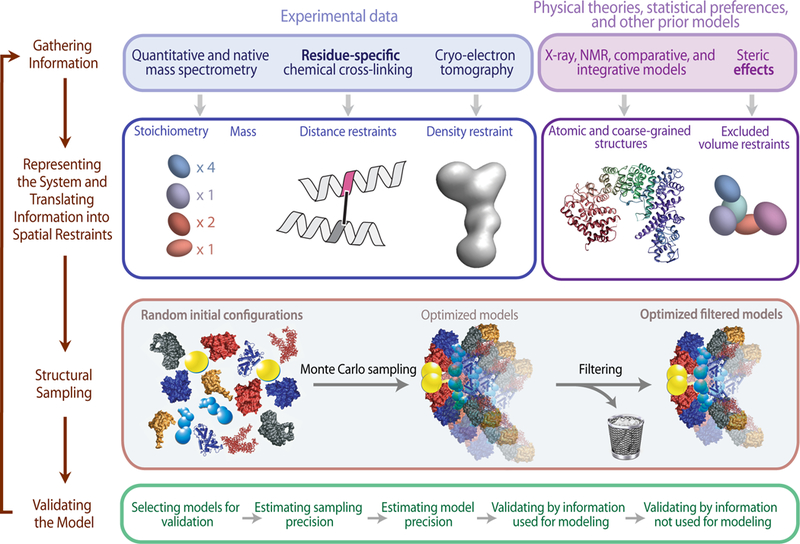
Figure 2. Workflow for Structural Analysis [2]
Cryo-EM employs a transmission electron microscope to examine samples at low temperatures by freezing them and maintaining them in a cryogenic state inside the microscope. This technique uses highly coherent electrons as a light source to penetrate the sample and its surrounding ice, causing scattering. The scattered signals are captured and processed by detectors and a lens system to reconstruct the sample's structure. Cryo-EM is essential for analyzing protein complexes frozen quickly to preserve their near-natural state. It is utilized not only for the structural determination of large biological molecules but also for smaller protein complexes, including membrane proteins. Cryo-EM complements other high-resolution structural biology techniques like X-ray diffraction (XRD) and NMR, offering advantages particularly in structure-based drug development, where traditional methods fall short.
Figure 3. Cryo-EM [3]
MtoZ Biolabs is equipped with an extensive cryo-EM experimental platform, including various cryo transmission electron microscopes and auxiliary equipment such as ultramicrotomes for both room temperature and low temperatures, high-pressure freezers, rapid grid cooling plunge freezers, vacuum coaters, and plasma cleaners. The facility also features a comprehensive high-performance computing cluster for microscopic image data storage and processing. Services offered include coating, plasma cleaning, negative dye sample preparation, rapid frozen sample preparation, and electron microscopy imaging and data collection, enabling detailed studies such as single particle analysis, electron tomography, and microcrystal electron diffraction. Our team can manage the entire process from protein expression and purification using both eukaryotic and prokaryotic systems, through to sample preparation and structural analysis using cryo-EM. MtoZ Biolabs offers a full spectrum of services from protein expression and sample optimization to sample preparation, cryo-EM analysis, and data interpretation, providing an all-in-one solution for structural analysis challenges.
References
[1] Mackay JP, Landsberg MJ, Whitten AE, Bond CS. Whaddaya Know: A Guide to Uncertainty and Subjectivity in Structural Biology. Trends Biochem Sci. 2017 Feb;42(2):155-167. doi: 10.1016/j.tibs.2016.11.002. Epub 2017 Jan 12. PMID: 28089412.
[2] Rout MP, Sali A. Principles for Integrative Structural Biology Studies. Cell. 2019 May 30;177(6):1384-1403. doi: 10.1016/j.cell.2019.05.016. PMID: 31150619; PMCID: PMC6810593.
[3] de la Cruz MJ, Eng ET. Scaling up cryo-EM for biology and chemistry: The journey from niche technology to mainstream method. Structure. 2023 Sep 27:S0969-2126(23)00332-5. doi: 10.1016/j.str.2023.09.009. Epub ahead of print. PMID: 37820731.
How to order?







