HCP Absolute Quantification Analysis Service
Host cell proteins (HCPs) are a very important class of impurities in the biopharmaceutical process. When host cells such as escherichia coli, yeast, mammalian cells, etc., are used to produce recombinant proteins or other biologics, their own proteins may mix with the target product. These residual HCPs can not only affect the safety and efficacy of drugs but may also provoke adverse reactions or immune responses. Therefore, precise quantitative analysis of HCPs is a crucial step in the development and production of biopharmaceuticals. The absolute quantitative analysis technique for HCPs based on mass spectrometry, by mixing a known concentration of standard HCPs with the sample to be tested and then comparing the response of the two via mass spectrometric analysis, can calculate the absolute concentration of HCPs in the sample and assess their potential impact on the drug, which is an important part of biopharmaceutical quality control.
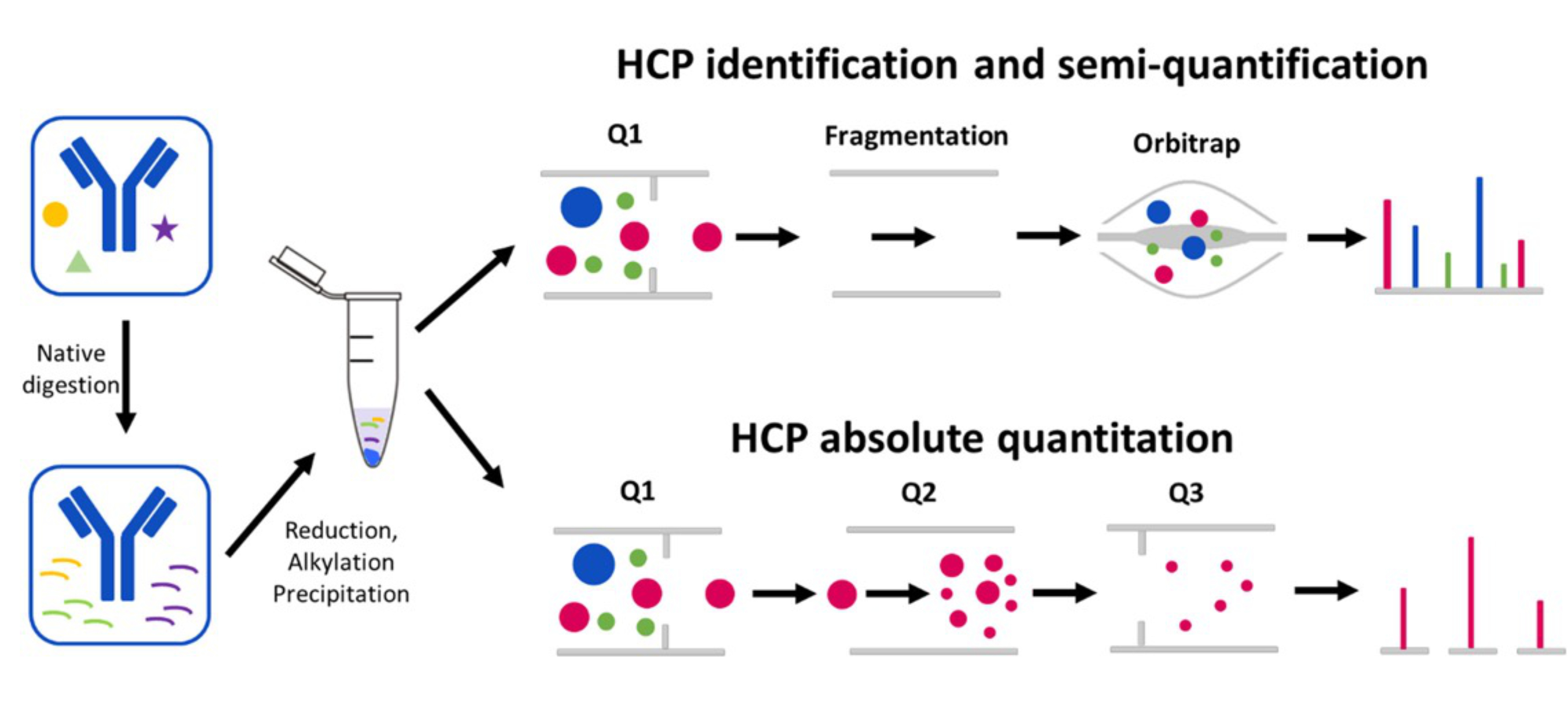
E, S. Y. J Pharm Sci. 2022.
Figure 1. HCPs Absolute Quantitative Analysis Process
Unlike traditional relative quantification methods, absolute quantification analysis can provide more accurate and specific information about the content of HCPs, offering a key quality control tool for the biopharmaceutical industry. MtoZ Biolabs uses the latest Thermo Orbitrap Fusion Lumos mass spectrometer combined with Nano-LC nanoliter chromatography technology, has developed an efficient HCPs absolute quantification platform, including a highly sensitive ELISA technology platform and an LC-MS/MS platform, providing you with compliant HCPs absolute quantification analysis services. Whether there are HCP antibodies or not, the total amount of all HCPs in the bioproduct can be absolutely quantified. For absolute quantification of certain specific HCPs (from a few to dozens), it can be achieved by synthetic heavy-labeled fingerprint peptides using PRM data acquisition mode. We provide you with a complete solution, monitoring the changes in HCPs content during the production process, thereby ensuring the quality and therapeutic efficacy of the drug.
Deliverables
In the technical report, MtoZ Biolabs will provide you with detailed technical information, including:
1. Experimental Procedures
2. Relevant Mass Spectrometry Parameters
3. HCPs Absolute Quantitative Analysis Details
4. Mass Spectrometry Images
5. Raw Data
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Host Cell Protein (HCP) Analysis Service
HCP Identification and Relative Quantitative Analysis Service
How to order?







