Impurity Profiling Service
- New Drug Development: Optimize synthetic pathways by identifying by-products and degradation products.
- Quality Control: Ensure process consistency and mitigate quality variability risks.
- Regulatory Submission: Support registration applications with robust impurity analysis data.
- Generic Drug Development: Provide reference data for impurity profiles in original formulations.
- Stability Studies: Monitor impurity changes over time to evaluate storage stability.
Impurity profiling is a specialized analytical technique aimed at identifying, quantifying, and characterizing impurities within pharmaceutical compounds. It provides insights into impurity origins, concentrations, chemical structures, and their potential impacts on drug safety and efficacy. As an essential element in pharmaceutical development and manufacturing, impurity profiling ensures that drug products meet stringent international regulatory standards while maintaining consistent quality across production batches. Impurity profiling plays a vital role in drug discovery, quality control, and process optimization, addressing a range of impurity types, including organic impurities, inorganic contaminants, and residual solvents.
MtoZ Biolabs offers end-to-end Impurity Profiling Service that spans from sample preparation to data interpretation, leveraging advanced analytical platforms and a team of seasoned experts to ensure precise and reliable results. Our solutions are tailored to meet the specific needs of pharmaceutical research, generic drug development, and active pharmaceutical ingredient (API) analysis, expediting research and development timelines.
Services at MtoZ Biolabs
To address diverse impurity analysis needs, MtoZ Biolabs provides specialized Impurity Profiling Service tailored to different impurity types, ensuring accurate and reliable results:
1. Organic Impurity Profiling
Utilizing high-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS) to identify by-products and degradation products formed during chemical synthesis.
2. Inorganic Impurity Profiling
Employing inductively coupled plasma mass spectrometry (ICP-MS) for precise detection of heavy metals, catalyst residues, and other inorganic contaminants.
3. Residual Solvent Analysis
Using gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS) to analyze trace residual solvents left after synthesis.
4. Unknown Impurity Identification
Applying nuclear magnetic resonance spectroscopy (NMR) and high-resolution mass spectrometry to determine the chemical structures of unidentified impurities.
Analysis Workflow
We follow a well-structured and robust workflow to ensure accuracy, reliability, and repeatability in Impurity Profiling Service:
1. Sample Reception and Assessment
Evaluate the sample and define analytical requirements based on impurity types and detection goals.
2. Sample Preparation
Optimize sample pre-treatment methods to ensure impurities are accurately captured during analysis.
3. Impurity Detection and Separation
Employ techniques like HPLC and GC for effective isolation and detection of impurities.
4. Data Interpretation and Reporting
Generate comprehensive impurity profiles, detailing impurity origins, content, and structures, along with actionable recommendations.
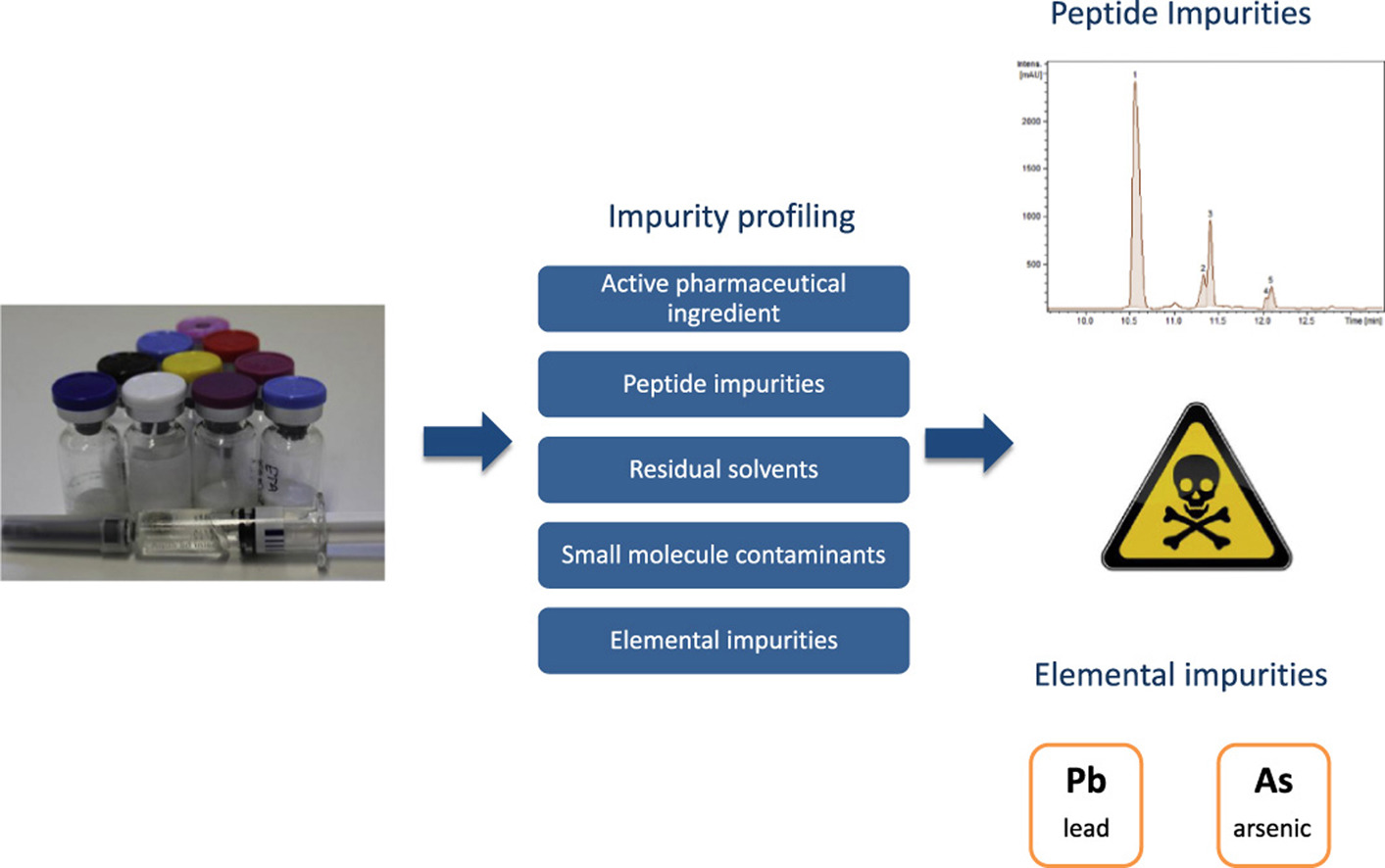
Janvier, S. et al. Talanta. 2018.
Figure 1. Impurity Profiling of Polypeptide Drugs
Service Advantages
1. Advanced Analysis Platform: MtoZ Biolabs offers a cutting-edge impurity profiling platform equipped with advanced instrumentation, including HPLC, LC-MS, GC-MS, ICP-MS, and NMR, ensuring reliable, fast, and highly accurate analysis results.
2. High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrate all impurity profiling data, providing clients with a comprehensive data report.
3. Integrated Solutions: From impurity identification to risk assessment, offering seamless end-to-end services.
4. One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
Applications
Impurity Profiling Service plays a pivotal role across various stages of pharmaceutical development and manufacturing:
Case Study
Case 1: Impurity Profiling Assists Avanafil Impurity Analysis and Toxicity Assessment
Using LC and LC-MS/MS, the study identified multiple impurities in Avanafil and assessed their potential safety risks through in vitro toxicity prediction. The analysis revealed key impurity sources and structures, highlighting the critical role of impurity profiling in risk evaluation.
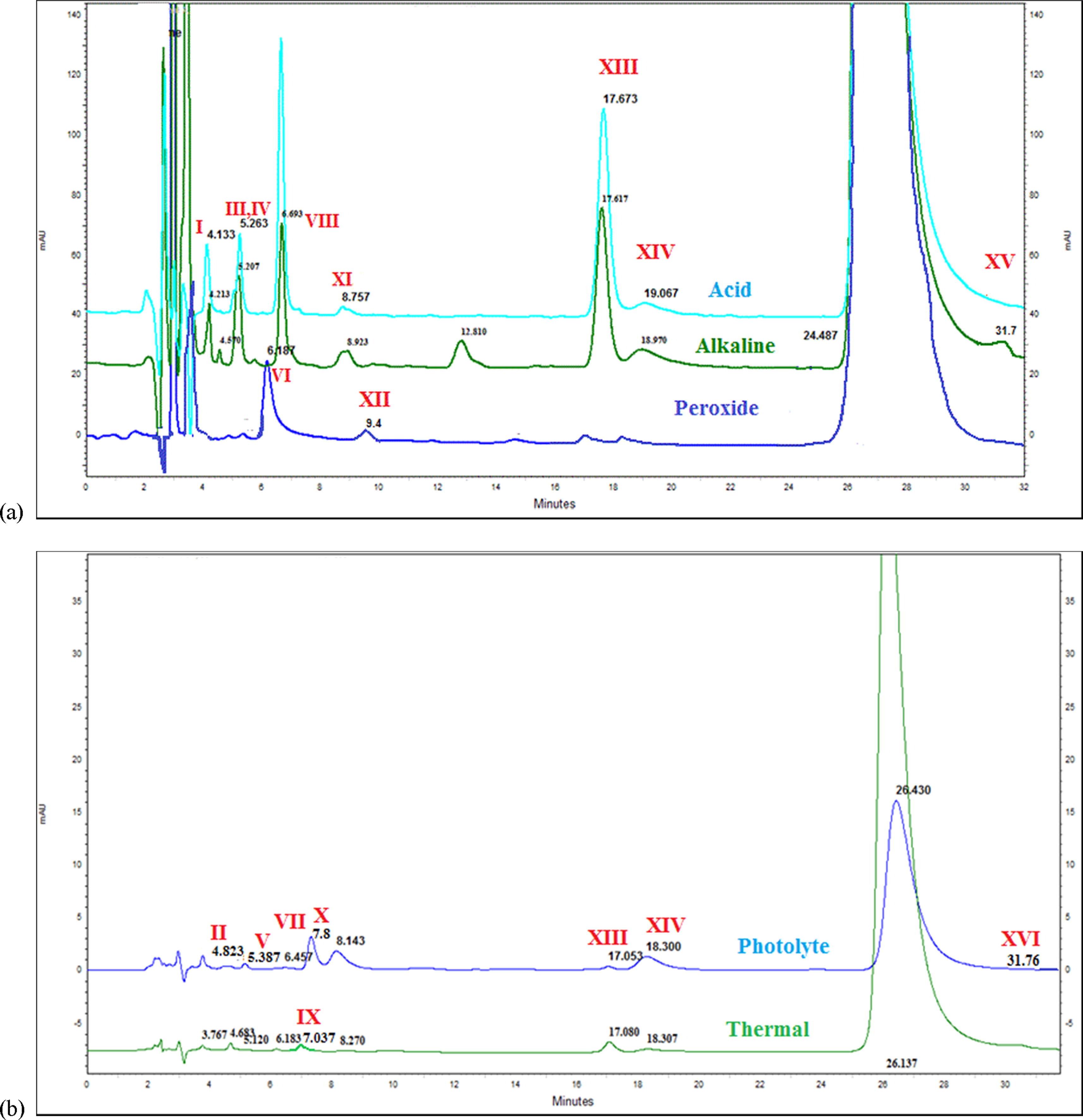
Mital, P. et al. Arab. J. Chem. 2020.
Case 2: Impurity Profiling Ensures the Quality and Safety of Esmolol Hydrochloride Injection
Comprehensive impurity analysis detected 20 impurities, including newly identified ones. Specific impurities were flagged for potential hepatotoxicity and mutagenicity, emphasizing the importance of rigorous impurity monitoring for product safety.
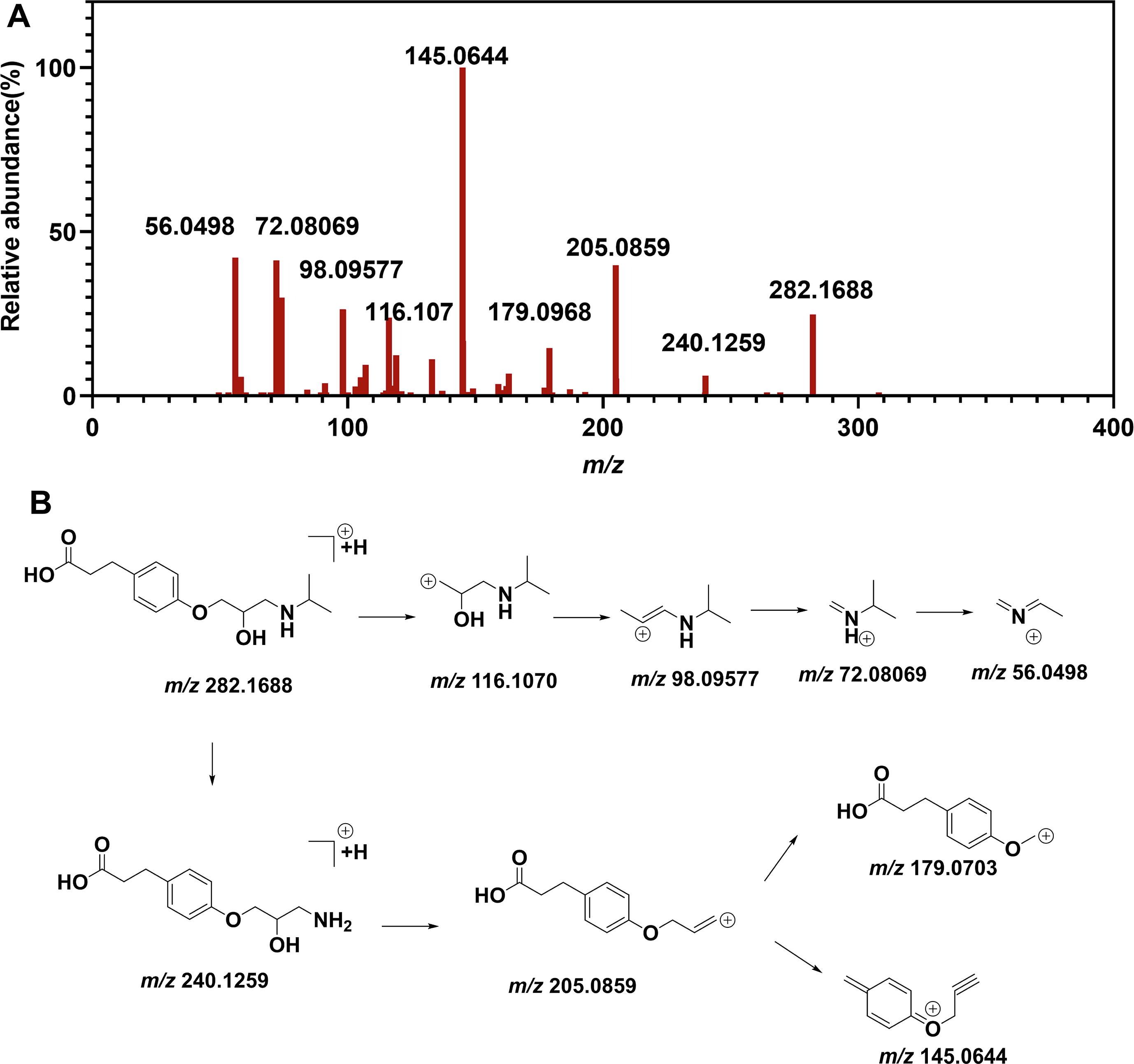
Zhang, W. et al. Arab. J. Chem. 2023.
At MtoZ Biolabs, we are committed to delivering reliable and precise Impurity Profiling Service. With a customer-centric approach and deep technical expertise, we offer tailored solutions to meet your specific needs. For further inquiries, please feel free to contact us.
How to order?







