Kynurenine Analysis Service
- Highly Efficient Sample Recovery and Processing
- Accurate and Reliable Detection and Quantification
- Capability to Handle Various Sample Types
- Customized Data Analysis Services
- Rapid Service Response and Cost-Effectiveness
- Animal tissue ≥ 100 mg
- Plant tissue ≥ 200 mg
- Serum/ Plasma ≥ 200 μL
- Urine ≥ 2 mL
The kynurenine pathway is the primary route for the catabolism of the amino acid tryptophan in mammals, leading to the production of several biologically active metabolites. The process begins with the oxidation of tryptophan by either tryptophan dioxygenase (TDO), primarily in the liver, or indoleamine dioxygenase (IDO) in peripheral tissues, producing N-formylkynurenine. This intermediate is then converted into kynurenine by formamidase.
Kynurenine serves as a key branching point in the pathway, leading to the production of several downstream metabolites. Kynurenine 3-monooxygenase (KMO) converts kynurenine into 3-hydroxykynurenine (3-HK), which is further metabolized by kynureninase to produce 3-hydroxyanthranilic acid (3-HAA). From 3-HAA, quinolinic acid, a neurotoxic metabolite, is formed, which is a precursor for nicotinamide adenine dinucleotide (NAD+) synthesis. Alternatively, kynurenine can be converted into kynurenic acid (KYNA) by kynurenine aminotransferases (KATs).
Kynurenic acid acts as a neuroprotective agent by antagonizing excitatory NMDA receptors and blocking alpha-7 nicotinic acetylcholine receptors. The balance between kynurenic acid (neuroprotective) and quinolinic acid (neurotoxic) is critical in regulating neuroinflammation and neurodegeneration. Dysregulation of this pathway has been linked to several diseases, including Alzheimer's disease, Parkinson's disease, and major depressive disorder. The pathway's metabolites also play significant roles in immune modulation, influencing T-cell activity and immune responses.
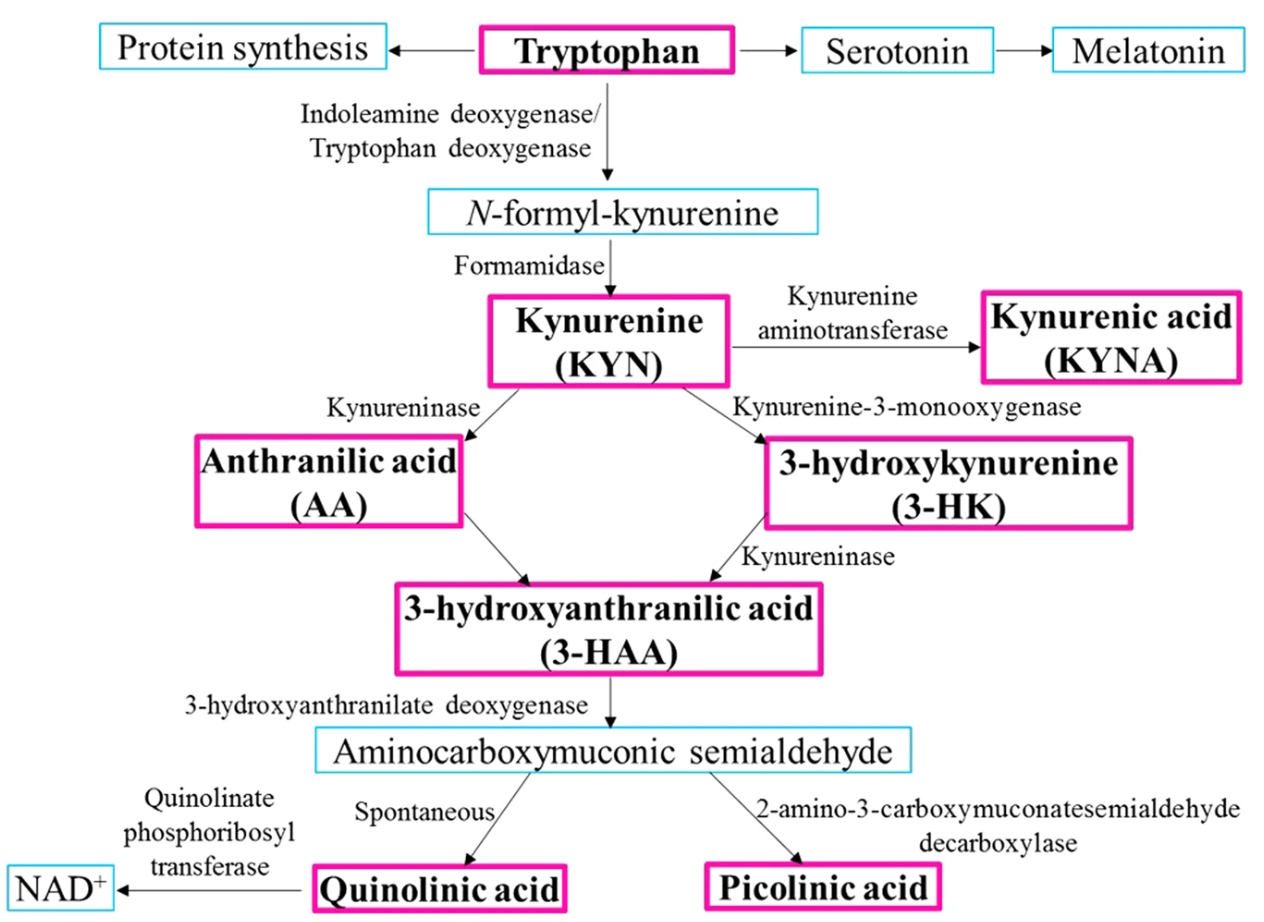
Figure 1. Schematic Diagram of The Kynurenine Pathway
Service at MtoZ Biolabs
Accurate quantification of kynurenine and its metabolites is essential for advancing research into these complex metabolic networks and their implications in disease. At MtoZ Biolabs, our Kynurenine Analysis Service utilizes state-of-the-art LC–MS/MS technology, ensuring high sensitivity and specificity for detecting kynurenine and its pathway metabolites across various biological samples, offering critical insights for biomedical research and clinical applications.
To provide a more comprehensive understanding of the metabolic network, we have developed a detailed Tryptophan Pathway Quantification Panel, covering key metabolites across the kynurenine, serotonin, and indole branches. This panel enables systematic profiling of tryptophan catabolism and supports studies in immunometabolism, neurobiology, and gut microbiome research.
Tabel 1. Tryptophan Pathway Quantification Panel
| No. | Metabolite | Abbreviation | Quantification Method | Pathway |
|---|---|---|---|---|
| 1 | L-Tryptophan | L-Trp | LC-MS/MS MRM (parent ion → indole fragment) | Starting substrate |
| 2 | N-Formylkynurenine | NFK | MRM (amide fragment monitoring) | Kynurenine pathway |
| 3 | Kynurenine | Kyn | MRM (aromatic ring fragment) | Kynurenine pathway |
| 4 | 3-Hydroxykynurenine | 3-HK | MRM (hydroxyl fragment transition) | Kynurenine pathway |
| 5 | Kynurenic acid | KYNA | MRM (decarboxylation fragment) | Kynurenine pathway |
| 6 | Anthranilic acid | AA | MRM (aromatic fragment ion) | Kynurenine pathway |
| 7 | 3-Hydroxyanthranilic acid | 3-HAA | MRM (dehydration ion transition) | Kynurenine pathway |
| 8 | Quinolinic acid | QA | MRM (carboxyl fragment ion) | Kynurenine pathway |
| 9 | Nicotinic acid (niacin) | NA | MRM (decarboxylation transition) | NAD⁺ biosynthesis |
| 10 | Nicotinamide | NAM | MRM (amide cleavage ion) | NAD⁺ biosynthesis |
| 11 | Nicotinamide mononucleotide | NMN | LC-MS/MS MRM (nucleotide phosphate ion) | NAD⁺ biosynthesis |
| 12 | Nicotinamide adenine dinucleotide | NAD⁺ / NADH | LC-MS/MS multi-MRM transition | NAD⁺ biosynthesis |
| 13 | 5-Hydroxytryptophan | 5-HTP | MRM (hydroxy-indole fragment) | Serotonin pathway |
| 14 | Serotonin (5-Hydroxytryptamine) | 5-HT | MRM (indole-amine fragment) | Serotonin pathway |
| 15 | N-Acetylserotonin | NAS | MRM (acetyl-amide fragment) | Serotonin pathway |
| 16 | Melatonin | MLT | MRM (indole fragment) | Serotonin pathway |
| 17 | Indole | IND | GC-MS or LC-MS/MS MRM | Indole pathway |
| 18 | Indole-3-acetic acid | IAA | MRM (decarboxylation ion) | Indole pathway |
| 19 | Indole-3-propionic acid | IPA | MRM (carboxylate ion) | Indole pathway |
| 20 | Indole-3-lactic acid | ILA | MRM (lactate fragment) | Indole pathway |
| 21 | Indole-3-acetamide | IAM | MRM (amide fragment) | Indole pathway |
| 22 | Picolinic acid | PA | MRM (decarboxylation ion) | Auxiliary metabolite |
| 23 | Nicotinamide riboside | NR | LC-MS/MS (nucleoside fragment) | Auxiliary metabolite |
Analytical platform: LC-MS/MS (MRM mode, dual polarity)
Internal standards: L-Trp-d₅, Kyn-d₄, 5-HT-d₄, MLT-d₄
Coverage: Kynurenine, Serotonin, and Indole branches
This integration naturally bridges the discussion of the kynurenine pathway with the broader tryptophan metabolism, positioning MtoZ Biolabs as a provider of end-to-end LC-MS/MS-based metabolic profiling solutions.
Analysis Workflow
Service Advantages
Applications
Kynurenine analysis is essential in numerous fields of study, including neuroscience, immunology, oncology and psychiatry.
Sample Submission Requirements
1. Sample Types
Serum, plasma, urine, tissues and other biological samples. For each sample, more than 3 materials of the same condition must be selected.
2. Sample Volume
3. Sample Preservation
Samples should be stored at -80°C to maintain stability.
Note: Please provide details on sample collection and handling.
Deliverables
1. Experimental Procedures
2. Relevant Liquid Chromatography and Mass Spectrometry Parameters
3. Detailed Information on Kynurenine
4. Raw Data
5. Custom Analysis Report
Contact us today to learn how our Kynurenine Analysis Service can support your research. At MtoZ Biolabs, we are committed to accelerating your scientific discoveries.
How to order?







