Peptide Drug Analysis Service
Peptides are compounds formed by multiple amino acids linked by peptide bonds and can be prepared via recombinant DNA expression, biological extraction, or chemical synthesis. Peptides represent a unique class of drugs with certain characteristics of small molecule drugs as well as properties of large proteins and other biologics. Most intrinsic signaling molecules are peptide-based, such as enzymes, hormones, and immune response mediators. Over 7,000 natural peptides have been identified. When endogenous secretion levels are insufficient or absent, peptides are crucial drugs that mimic various natural pathways and hormone therapies.
Drugs approved by the FDA can roughly be divided into small molecules, large molecules (biologics), and peptides (mid-sized drugs). Among all FDA-approved drugs, small molecule drugs account for 83%, biologics account for 11%, and peptide drugs 6%. In recent years, the demand for peptide therapies has significantly increased, leading to over 100 peptide drugs being approved.
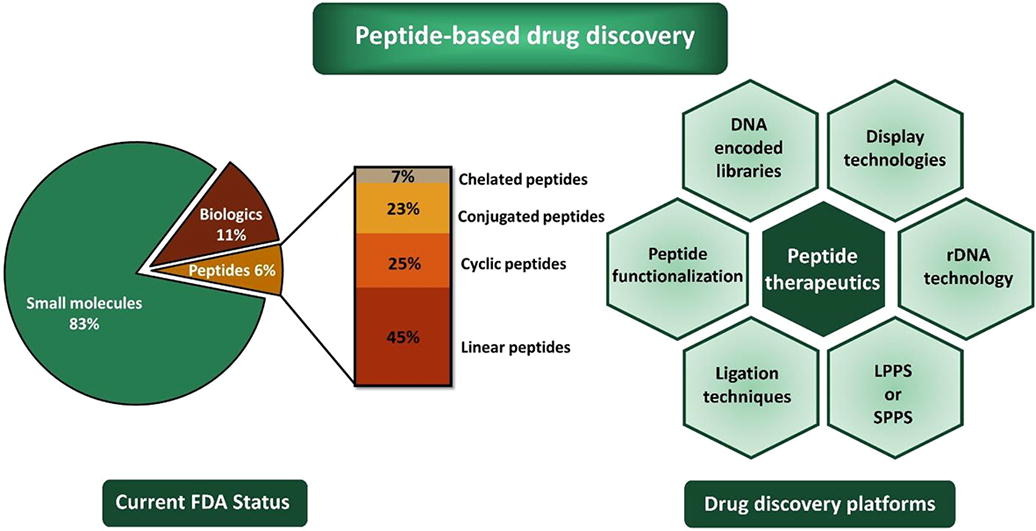
Figure 1. Peptide-Based Drug Discovery [1]
Advantages and Limitations of Peptides as Therapeutic Drugs
1. Advantages
(1) General advantages: High efficiency, strong selectivity, and less toxicity.
(2) Compared to small molecule drugs: Due to their short half-life, peptides tend to accumulate less in tissues, thus possessing higher target specificity and therefore fewer side effects.
(3) Compared to other biologics: Peptides have greater transmembrane permeability and lower immunogenicity. They are unique due to their small structure, easy to synthesis, and more stable and easy to store. They are cost-effective due to their smaller size and advanced synthesis strategies.
2. Limitations
(1) Metabolic instability due to proteolytic cleavage
(2) Rapid clearance due to enzymatic metabolism
(3) Low oral bioavailability due to metabolic instability
(4) Low solubility of some peptides due to their strong hydrophobicity
(5) Poor membrane permeability due to the presence of charged and hydrophilic or polar amino acids
Strategies to Overcome Limitations Associated with Peptide
The pharmacokinetic of peptides includes thier absorption, half-life, metabolism, and bioavailability, which can be modulated through various structural modifications. Common methods for peptide structural adjustment include:
1. Backbone Modification, Removing Dipeptides, Azapeptides, Thiopeptides, Retro-inverso Peptides, Fluoroalkenes, and Replacing Amide Bonds with Triazoles or Oxazolidinone
2. N- or C-terminal Modification, Such as N-alkylation, N-heterocyclic Coupling, C-esterification, or Amidation
3. Macrocyclization of Peptides, Using Head-to-tail, Side-chain-to-side-chain, Head-to-side-chain, and Side-chain-to-tail Methods and Ring-closing Metathesis, Cross-coupling Reactions, and Linking Strategies
4. Peptide Creation, Replacing Natural Amino Acids with Non-Natural or Modified Amino Acids, Synthesizing Peptidomimetics, and Through Polyethylene Glycol (PEG)ylation or Lipidation
5. Peptides Substitution with Glycosyl or Lipid Groups Can Enhance the Permeability and Bioavailability of Peptides
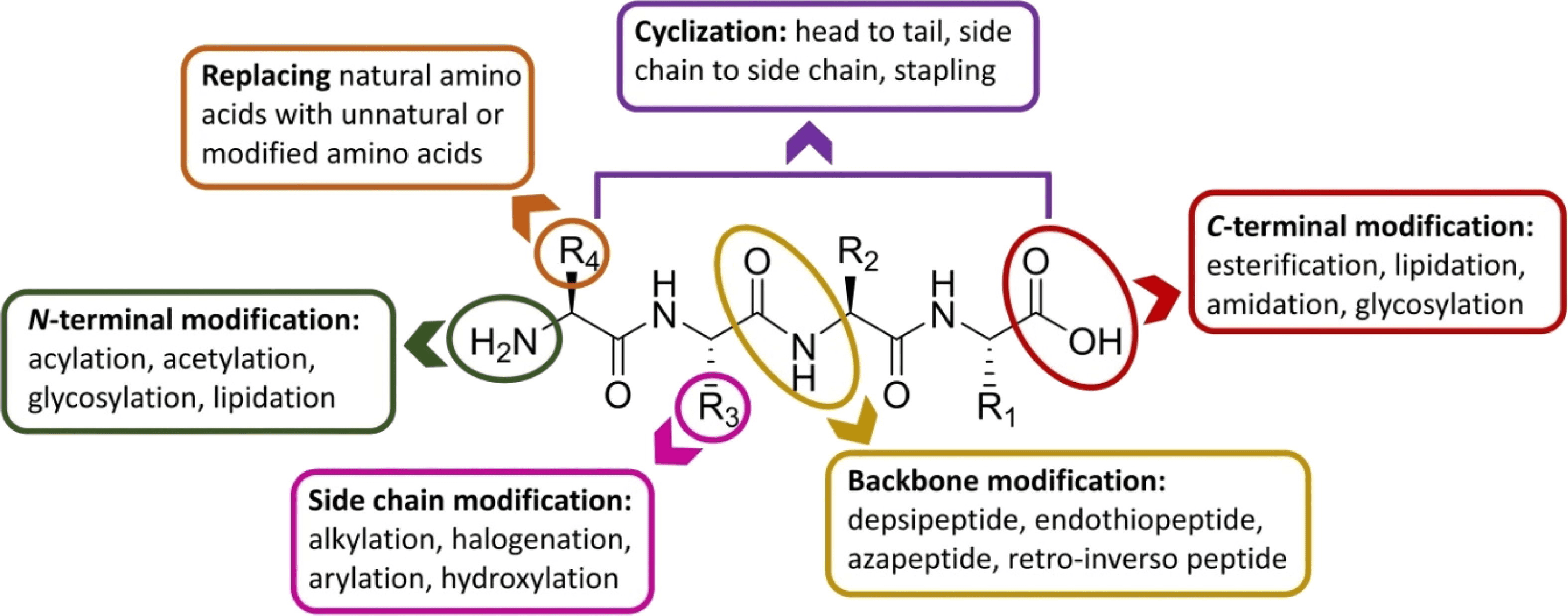
Figure 2. Strategies to Overcome the Limitations Associated with Peptide Use and to Improve Their Drug-like Properties [1]
Testing Content Required by Policies and Regulations
FDA quality standards for peptide drugs cover various aspects, including but not limited to the chemical, physical, and biological properties of the drug, as well as quality control measures during production. Here are some specific quality requirements and standards:
1. Chemical and Physical Properties
(1) Defined Chemical Structure: The amino acid sequence, molecular weight, stereochemistry, and any modifications (such as PEGylation) of the peptide drugs must be clearly defined.
(2) Purity and Impurity Control: The purity of peptide drugs must meet the regulatory standards, and levels of impurities (including peptide-related and non-peptide-related impurities) must be controlled within safe limits.
(3) Stability: Peptide drugs must remain stable under expected storage and usage conditions, and stability studies should include accelerated and long-term stability tests.
2. Production Process
(1) cGMP Compliance: The production of peptide drugs must adhere to current good manufacturing practices (cGMP).
(2) Production Consistency: The production process should ensure batch-to-batch consistency, including synthesis, purification, and formulation steps.
(3) Validation and Verification: Critical parameters and control measures of the production process must be validated and verified.
3. Quality Control Testing
(1) Detailed quality control testing plan, including tests on raw materials, intermediates, and final products.
(2) Validation of Testing Methods: All quality control testing methods must be validated to ensure the accuracy and reliability of results.
4. Bioequivalence and Preclinical Data
(1) For generics, its bioequivalence to the reference drug must be demonstrated.
(2) Preclinical Data: For new drugs, preclinical study data on pharmacology, toxicology, and pharmacokinetics (PK) must be provided.
5. Labeling and Instructions
The labeling and instructions of the drug should contain all relevant information about safety, efficacy, and usage.
6. Immunogenicity Assessment
For peptide drugs that may have immunogenic potential, an immunogenicity risk assessment must be conducted, and clinical immunogenicity evaluations performed if necessary.
7. Drug Interactions
Assessment of potential drug interactions of peptide drugs, especially their effects on CYP enzymes and drug transport proteins.
8. Regulatory Pathway
Depending on the drug's characteristics and development stage, an appropriate regulatory pathway, such as ANDA (abbreviated new drug application) or NDA (new drug application), should be selected.
9. Combination Products
For combination drugs containing peptide or protein components, quality assessment may be a collaborative effort among multiple sub-offices of office of pharmaceutical quality (OPQ).
10. Change Management
Any changes in the production process must be properly managed, and the FDA notified when necessary.
ANDAs for Certain Highly Purified Synthetic Peptide Drug Products That Refer to Listed Drugs of rDNA Origin
1. Introduction
This guideline aims to provide advice to assess whether synthetic peptide drug products referring to approved rDNA origin peptide drugs (such as glucagon, liraglutide, nesiritide, teriparatide, and teduglutide) are suitable for submission as ANDAs. The ANDA should demonstrate that the synthetic peptide has the same active ingredient as the rDNA-origin drug and is comparable in terms of purity, safety, and efficacy.
2. Background
According to the Federal Food, Drug, and Cosmetic Act (FD&C Act), the FDA defines any α-amino acid polymer containing 40 or fewer amino acids as a peptide, not a protein. Approval of an ANDA requires demonstrating that the proposed generic drug is the same as the reference listed drug (RLD) in terms of active ingredients, conditions of use, dosage form, route of administration, strength, and labeling (with certain allowable differences) and is bioequivalent to RLD.
3. Scientific Considerations for ANDA Submission
(1) The Same Active Ingredient: Determined through physicochemical properties and biological assessments (such as clinical PK and/or pharmacodynamics studies).
(2) Impurities: During the review of an ANDA, the FDA considers the types and amounts of impurities present in the proposed generic drug compared to the RLD. Synthetic peptides should not contain higher levels of impurities than the RLD; any impurities, including new ones, should be reasonably explained to ensure that the generic drug does not pose a greater safety risk than the RLD, including immunogenic risks.
Clinical Pharmacology Considerations for Peptide Drug Products Guidance for Industry DRAFT GUIDANCE
1. Introduction
This guidance provides recommendations for clinical pharmacology considerations in the development of peptide drug products, including the impacts of hepatic impairment, drug-drug interactions (DDI), QT interval prolongation risks, and immunogenicity risks on the PK, safety, and efficacy of peptide drug products.
2. Definition of Peptide Drug Products
A peptide is defined as a polymer composed of 40 or fewer amino acids. If a peptide meets the definition of a drug but does not meet the statutory definitions of a "biologics" or "device," it is regulated as a drug under the Federal Food, Drug, and Cosmetic Act (FD&C Act).
3. Clinical Pharmacology Evaluation
(1) Immunogenicity Risk Assessment: Most peptide drug products have potential immunogenicity, thus requiring an assessment of immunogenicity risk for all peptide drug products.
(2) Impact of Hepatic Impairment: Although peptide drug products are generally metabolized extensively by proteases and peptidases, assessing the its impact of liver on the PK may be important in certain situations.
(3) Drug Interaction Assessment: Peptide drug products generally are not metabolized by CYP enzymes and are therefore not affected by CYP enzyme inhibitors or inducers. However, certain structurally modified peptides (such as cyclic peptides) may be subject to CYP enzyme-mediated metabolism and transporter-mediated disposition.
(4) Characterization of QT Interval Prolongation: QT studies, formally known as QT interval studies, are clinical trials conducted during drug development to assess the impact of new drugs on the cardiac QT interval. Peptides composed only of natural amino acids have a low likelihood of direct ion channel interactions and usually do not require thorough QT studies unless mechanistic considerations or preclinical study data indicating a potential risk of arrhythmias.
Peptide Drug Analysis Solutions
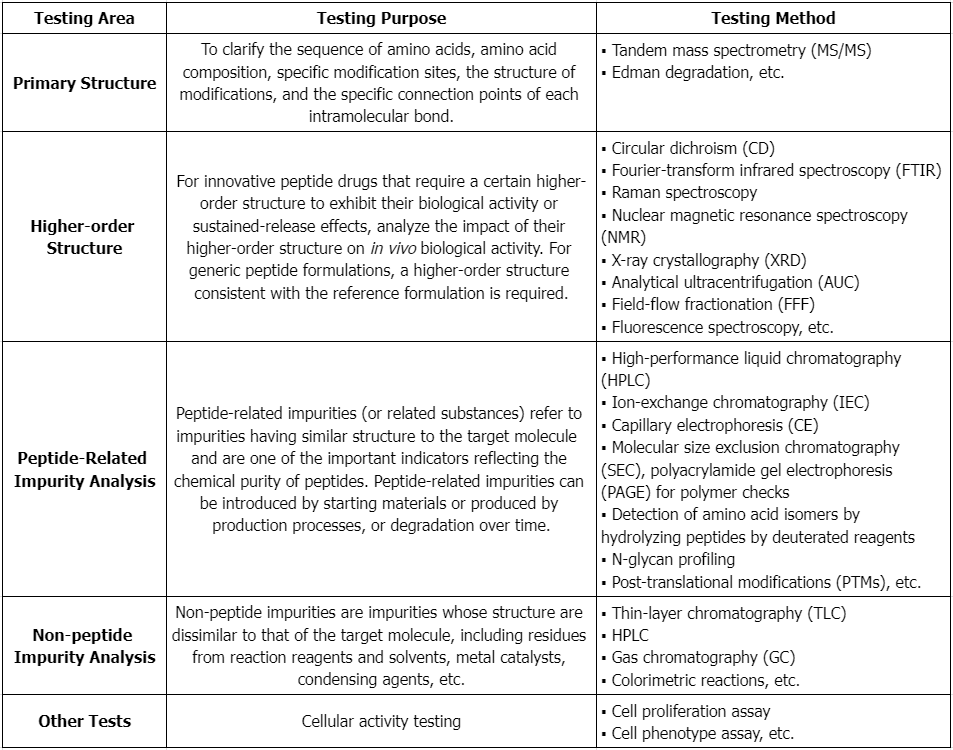
Service Advantages
1. Provides Professional and Robust Services, Covering Various Aspects of Peptide Drug Testing
2. Offers High Cost-Effectiveness, with Short Experimental Cycles and Complete, Reliable Reports
3. Equipped with Multiple High-Resolution Mass Spectrometers and Other Large-Scale Instruments
Case Study
1. High-Performance Liquid Chromatography-High Resolution Mass Spectrometry for Peptide Drug Quality Testing (Salmon Calcitonin, Bivalirudin, and Exenatide) [2]
A method using liquid chromatography-high resolution mass spectrometry (LC-HRMS) was developed with salmon calcitonin, bivalirudin, and exenatide as model systems to assess the suitability of this method for monitoring the quality of peptide drug products. The calcitonin and its related impurities ranged from 0.1 to 10 μM, (R2 values for salmon calcitonin), Glu14-calcitonin, and acetyl-calcitonin of 0.995, 0.996, and 0.993, respectively. Intra-assay precision in terms of relative standard deviation (%RSD), was less than 10% at all tested concentrations. The method's accuracy exceeded 85% as measured by spiking 0.1%, 0.3%, and 1% of Glu14-calcitonin and acetyl-calcitonin into stock calcitonin solutions. The detection limits for calcitonin, Glu14-calcitonin, and acetyl-calcitonin were 0.02, 0.03, and 0.04 μM, respectively, indicating that impurities as low as 0.1% (0.1 μM) of the drug product API concentration (107 μM) could be detected. Method validation studies for bivalirudin and exenatide drug products showed similar results in selectivity, sensitivity, precision, and linearity as those for salmon calcitonin. Another benefit of using the LC-HRMS method is that it enables determination of amino acid composition, peptide sequence, and quantification of impurities in a single experimental run, even during co-elution. LC-HRMS represents a promising approach for peptide quality control, including the measurement of any peptide-related impurities.
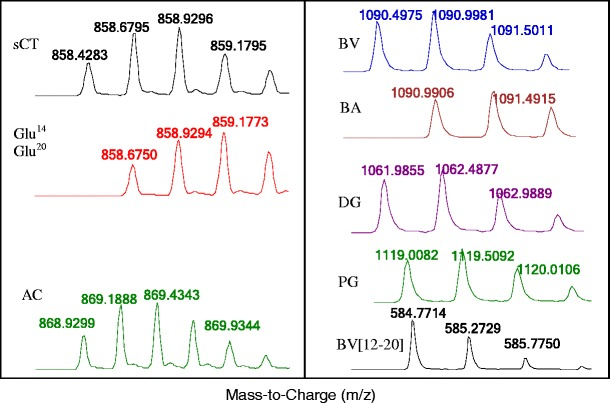
Figure 3. High-Resolution Mass Spectrometry of Salmon Calcitonin, Bivalirudin, and Their Related Impurities
2. Liquid Chromatography-High Resolution Tandem Mass Spectrometry for Impurity Identification and Quantification of the Peptide Hormone Angiotensin I, and the Metrological Impact on Value Assignment by Amino Acid Analysis [3]
Liquid chromatography-high resolution tandem mass spectrometry (LC-hrMS/MS) is a crucial technique for detecting, identifying, and quantifying structurally related impurities in peptide materials. A study described experiments with the model peptide hormone angiotensin I (ANG I). Degradation products produced by ANG I after storage at elevated temperatures were screened. Peptide fragments such as ANG II or ANG III were identified by comparing measured mass values with calculated mass values. Using data-dependent acquisition (DDA) techniques, ANG II and other peptide fragments were detected and characterized as major impurities in a LC-hrMS/MS analytical run. Subsequent quantification was performed using external calibration, estimating the mass fraction of major impurities in candidate reference materials at 10.4 mg/g. Failure to correct these impurities would result in a 1% error in peptide concentration measurements in solutions determined by amino acid analysis techniques.
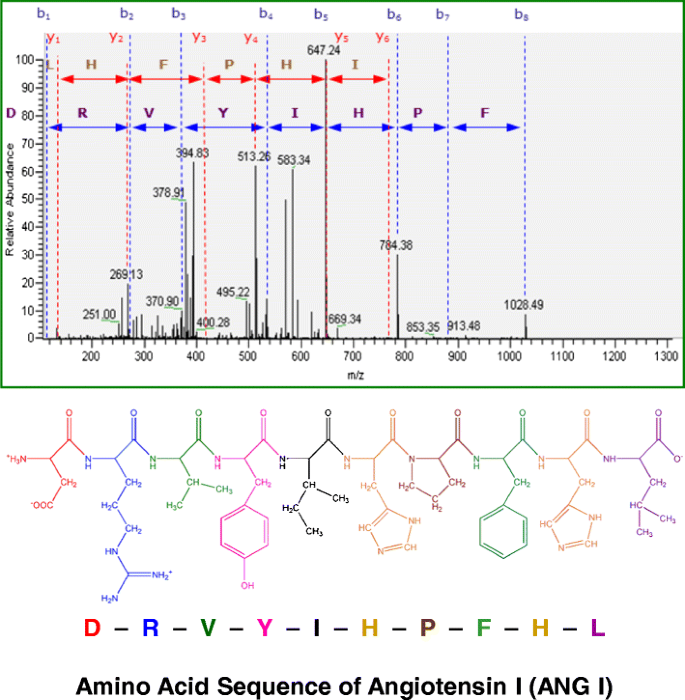
Figure 4. Liquid Chromatography-High Resolution Tandem Mass Spectrometry Analysis of The Peptide Hormone Angiotensin I
References
[1] Sharma K, Sharma KK, Sharma A, Jain R. Peptide-based drug discovery: Current status and recent advances. Drug Discov Today. 2023 Feb;28(2):103464. doi: 10.1016/j.drudis.2022.103464. Epub 2022 Dec 5. PMID: 36481586.
[2] Zeng K, Geerlof-Vidavisky I, Gucinski A, Jiang X, Boyne MT 2nd. Liquid Chromatography-High Resolution Mass Spectrometry for Peptide Drug Quality Control. AAPS J. 2015 May;17(3):643-51. doi: 10.1208/s12248-015-9730-z. Epub 2015 Feb 26. PMID: 25716148; PMCID: PMC4406950.
[3] Stoppacher N, Josephs RD, Daireaux A, Choteau T, Westwood SW, Wielgosz RI. Impurity identification and determination for the peptide hormone angiotensin I by liquid chromatography-high-resolution tandem mass spectrometry and the metrological impact on value assignments by amino acid analysis. Anal Bioanal Chem. 2013 Oct;405(25):8039-51. doi: 10.1007/s00216-013-6953-7. Epub 2013 May 25. PMID: 23708692.
How to order?







