Protein Lactylation Modification Analysis Service
In cell biology and biochemistry, post-translational modifications (PTMs) critically influence the stability, activity, localization, and functions of proteins. Among these, lactylation stands out as a significant PTM. This modification can occur on any protein residue with a free amino group, such as lysine, arginine, and tyrosine. During this process, lactate reacts with these amino acids to form an ester linkage, thereby altering protein structure and function. This change impacts several biological processes, including cell signaling, gene expression, and metabolism. Accurate identification and characterization of lactylation sites are essential for understanding their biological implications and for the development of related applications.
Mass spectrometry is crucial for identifying lactylated proteins and studying their properties. This technique allows for precise determination of lactylation sites and explores the modification's impact on protein structure and function. Additionally, mass spectrometry is also used to screen for new lactylation sites, providing deeper insights into protein lactylation studies. Moreover, research on lactylation modification has a broad application value, including but not limited to understanding the structure and function of proteins, studying the pathogenesis of diseases, and the development of novel drugs, and helping us understand the complexity and dynamics of biological systems.
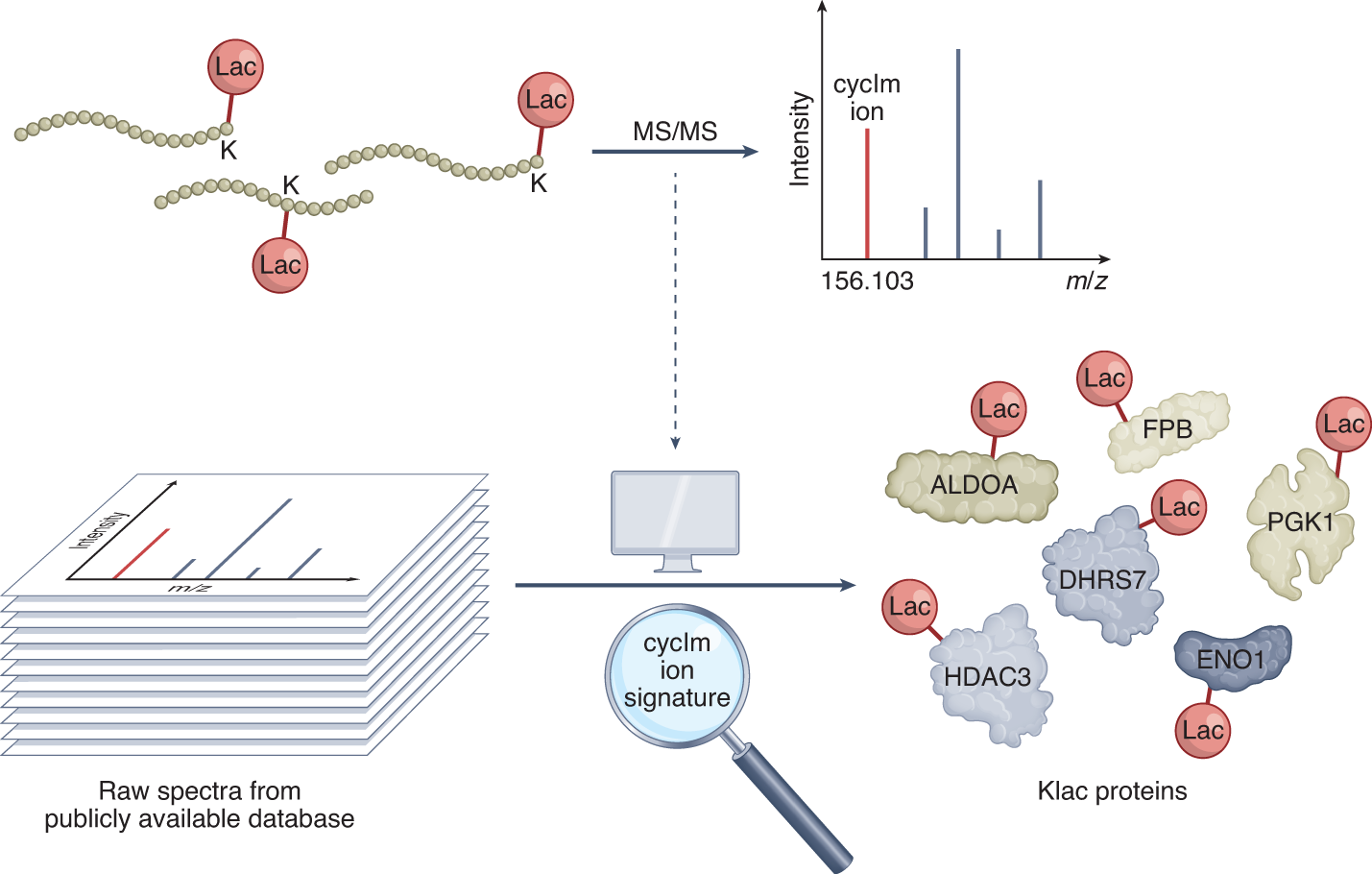
Wu, X. et. al. Nat. Methods. 2022.
Figure 1. Mass Spectrometric Analysis of Protein Lactylation Modification
At MtoZ Biolabs, using the latest Thermo's Orbitrap Exploris 240 mass spectrometer in combination with Nano-LC technology, we have developed a precise analytical platform for protein lactylation. This platform is adept at pinpointing lactylation sites and assessing their dynamic changes under various conditions, thereby revealing their effects on protein structure and function. Whether you aim to investigate the fundamental mechanisms of lactylation or its role in disease progression, MtoZ Biolabs offers comprehensive, efficient, and tailored analytical solutions. Contact us for more information on our protein lactylation modification analysis services.
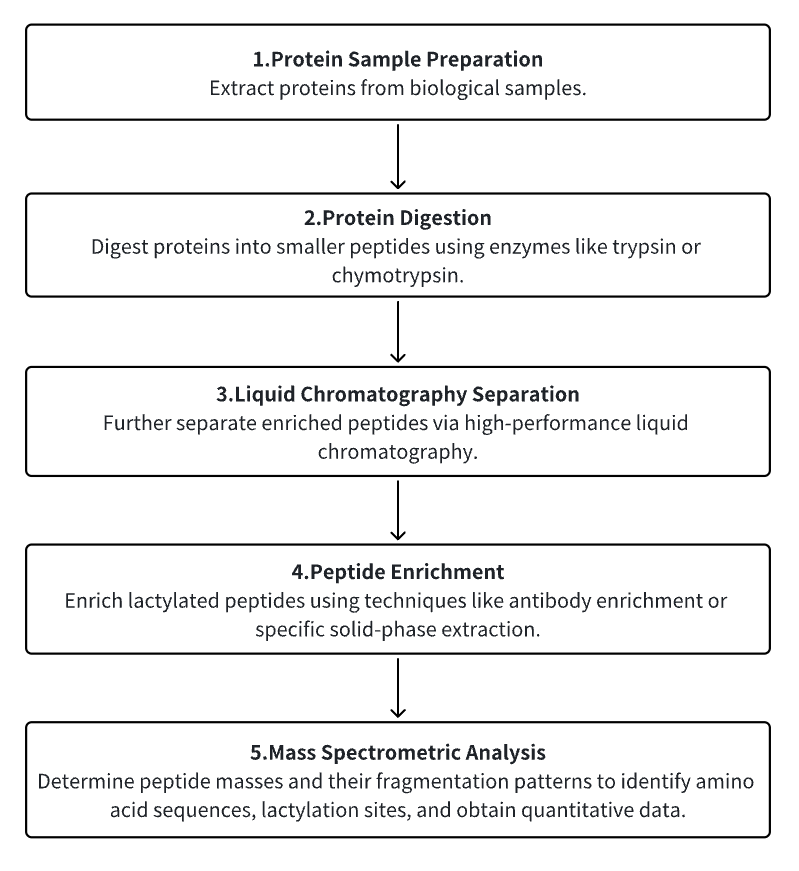
Analysis Workflow
1. Protein Sample Preparation
Extract proteins from biological samples.
2. Protein Digestion
Digest proteins into smaller peptides using enzymes like trypsin or chymotrypsin.
3. Liquid Chromatography Separation
Further separate enriched peptides via high-performance liquid chromatography.
4. Peptide Enrichment
Enrich lactylated peptides using techniques like antibody enrichment or specific solid phase extraction.
5. Mass Spectrometric Analysis
Determine peptide masses and their fragmentation patterns to identify amino acid sequences, lactylation sites, and obtain quantitative data.
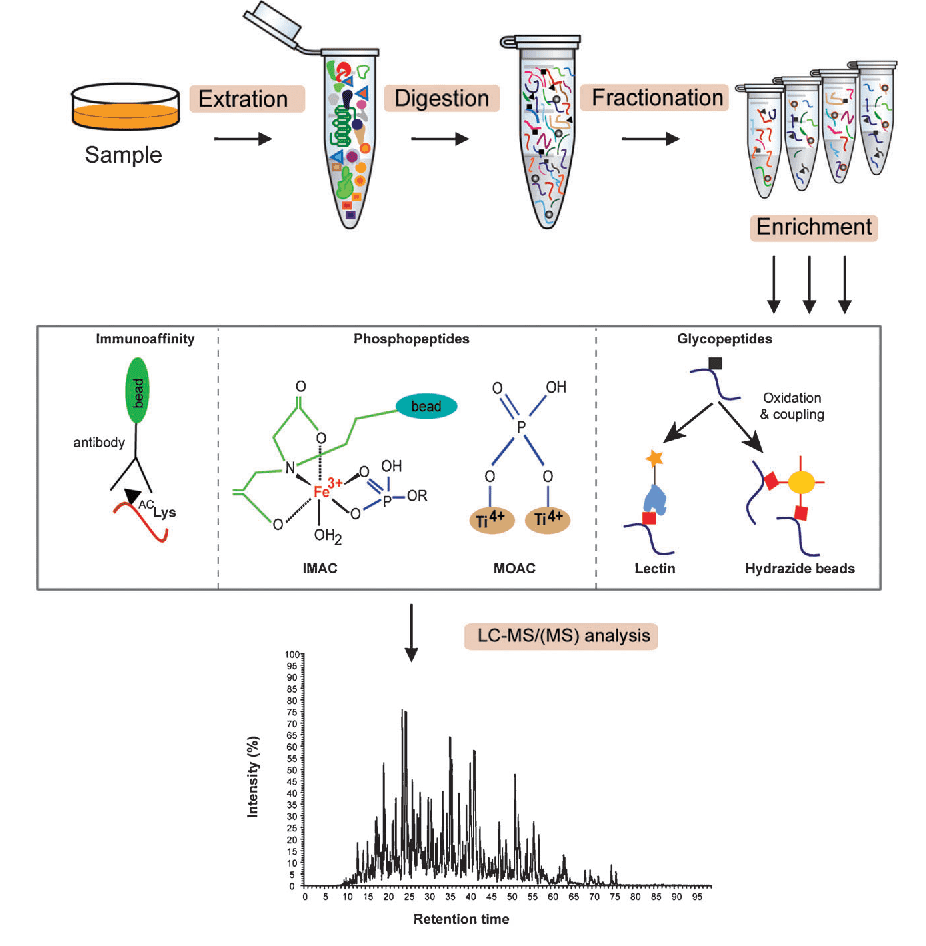
Angel, T. E. et. al. Chem. Soc. Rev. 2012.
Figure 2. Lactylation Modification Analysis Process
Service Advantages
1. Sensitivity and Accuracy
Detect lactylation modifications with high precision even at low abundances.
2. Localization of Modification Sites
Identify lactylation on proteins and precisely determine the affected amino acid residues.
3. Quantitative Capabilities
Assess the relative or absolute abundance of lactylation modifications to understand their biological impacts.
4. High-throughput Capability
Identify and quantify numerous lactylation sites in a single experiment.
5. Compatibility
Combine with other techniques like immunoprecipitation and bioinformatics for more comprehensive analyses.
Deliverables
In the technical report, MtoZ Biolabs will provide you with detailed technical information, including:
1. Experimental Procedures
2. Relevant Mass Spectrometry Parameters
3. Detailed Information on Protein Lactylation Modifications
4. Mass Spectrometry Images
5. Raw Data
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Post-Translational Modifications Proteomics Service
How to order?







