Protein Lactylation: Principle, Mechanism and Detection
Discovery of Protein Lactylation
For a long time, lactate was considered a “waste product” of cellular metabolism. In the classical glycolytic pathway, cells under hypoxia or high metabolic demand convert glucose into pyruvate via glycolysis. When oxygen is insufficient, pyruvate cannot enter the mitochondrial TCA cycle and is instead reduced to lactate to regenerate NAD⁺, allowing glycolysis to continue. Although this process maintains energy production, it also leads to lactate accumulation. Consequently, lactate has long been labeled a byproduct of anaerobic metabolism and thought to require clearance through transport or hepatic metabolism to maintain homeostasis.
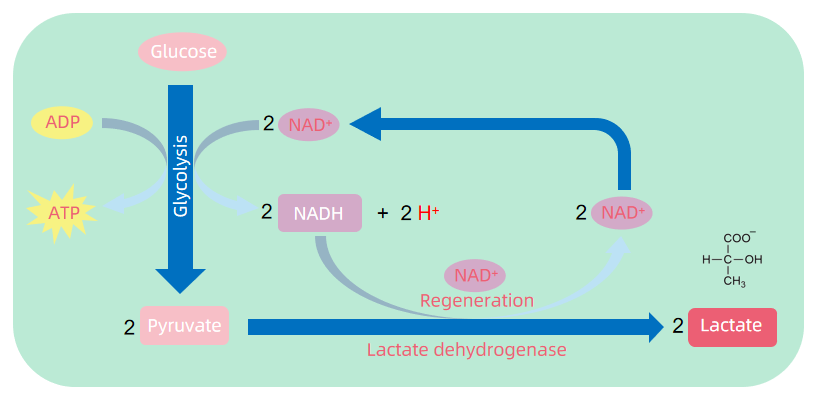
Figure 1. The Process of Aerobic Glycolysis
However, recent studies have shown that lactate is far from useless. It serves as a versatile metabolic signal and substrate. Lactate can act as a metabolic fuel shuttled between cells and tissues and also functions through specific receptors (e.g., GPR81) to mediate signaling events in immune, neurological, and endocrine systems. More importantly, lactate can serve as a carbon donor in post-translational modification, modifying lysine residues via acylation and giving rise to a novel regulatory mechanism—protein lactylation—that directly links metabolic status with gene expression.
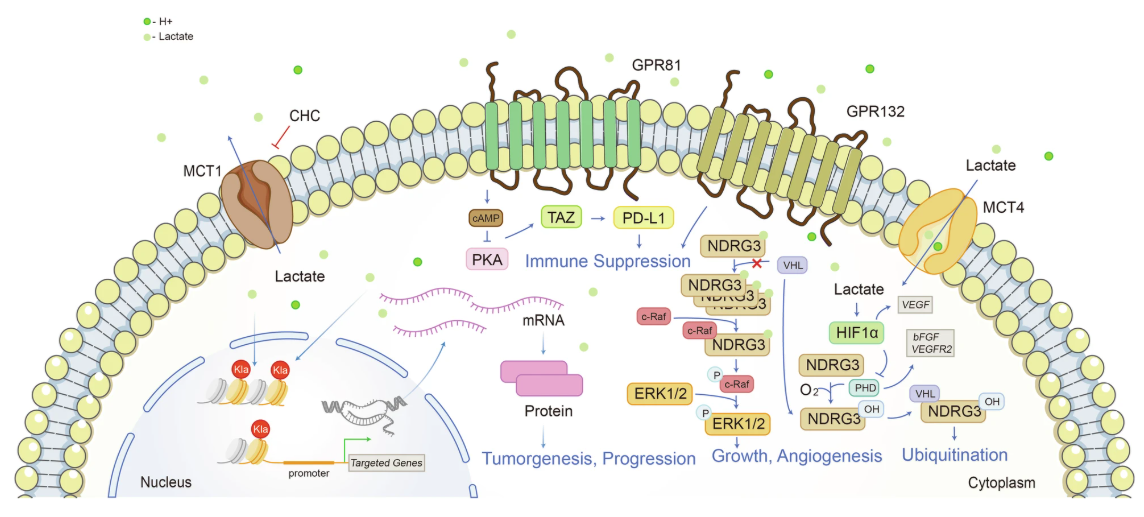
Chen, J. et al. Signal Transduct Target Ther. 2025.
Figure 2. Lactate is an Intracellular and Extracellular Signal Transducer of Great Significance
The discovery of protein lactylation has opened new avenues for understanding the interplay between metabolism, epigenetics, and cellular function, with implications for disease mechanisms and therapeutic development.
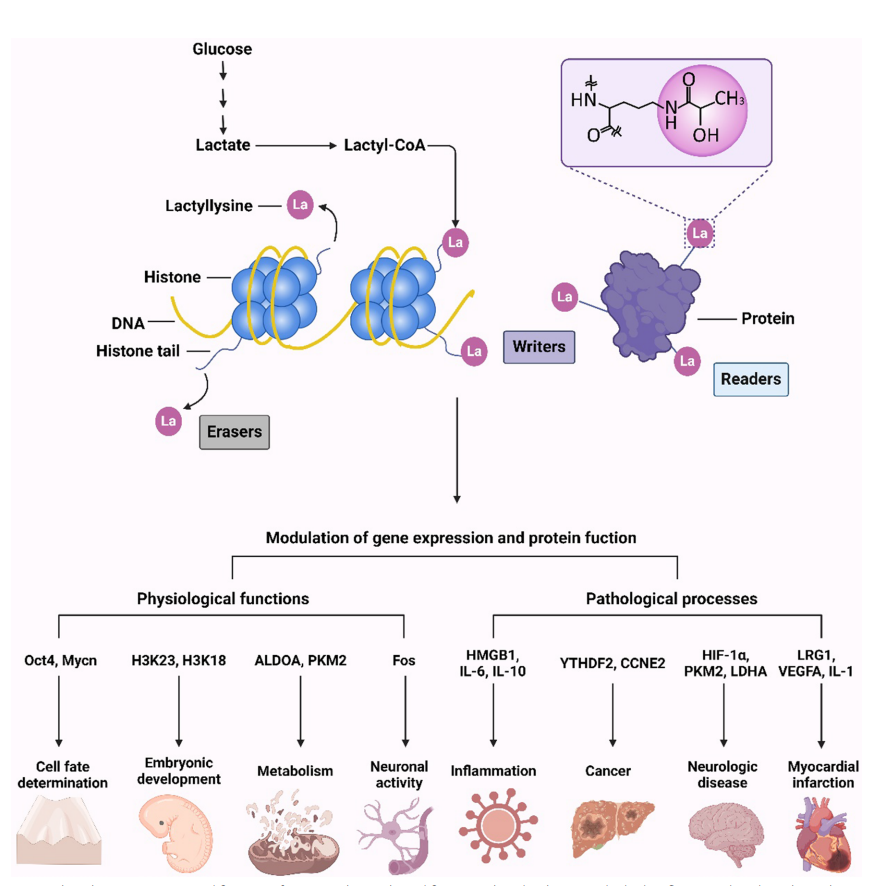
Hu Y, et al. Clin Epigenetics. 2024.
Figure 3. Protein Lactylation Regulates the Gene Expression and Protein Function
Services you may be interested in:
The Mechanism of Lactylation
Protein lactylation is a novel lysine-targeted post-translational modification, in which a lactyl group (–CO–CH₃OH) is covalently attached to the ε-amino group of lysine residues via an amide bond. This process is primarily driven by the metabolic intermediate lactoyl-CoA, serving as the acyl donor under enzymatic or non-enzymatic conditions. The production of lactoyl-CoA may result from enhanced glycolysis, lactate accumulation, or metabolic bypass involving CoA derivatives.
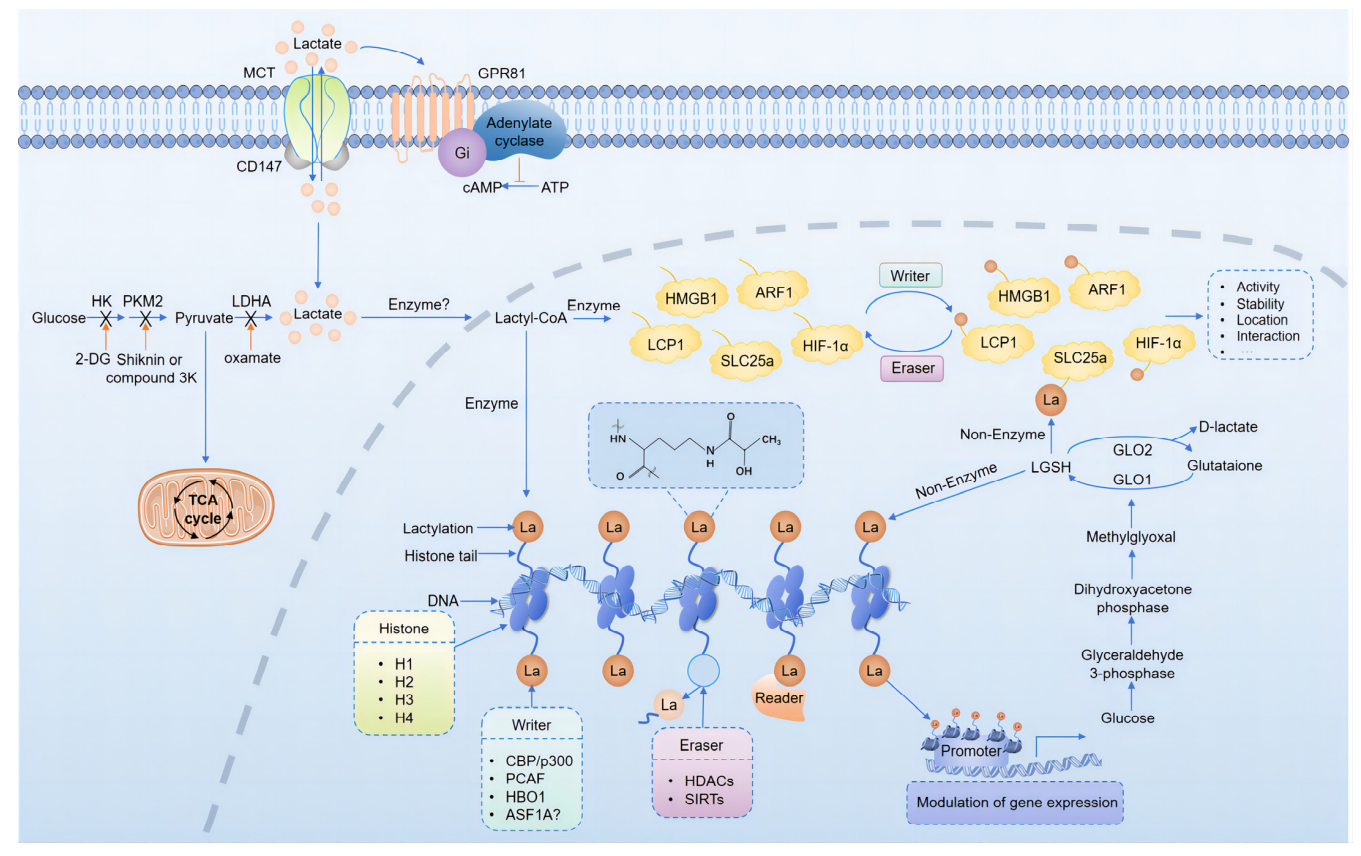
Liu J, et al. Biomolecules. 2024.
Figure 4. Mechanisms and Regulation of Lactylation
Although specific lactyltransferases ("writers") have not yet been definitively identified, studies suggest that enzymes such as p300/CBP, which are known histone acetyltransferases (HATs), may also catalyze lactylation under certain metabolic conditions. p300 has been observed to bind lactoyl-CoA in high-lactate environments and modulate lactylation at specific lysine residues. High-throughput screening efforts are underway to identify other potential writers, although none have been conclusively validated to date.
In contrast, research on “erasers” (enzymes that remove lactyl groups) remains preliminary. Some histone deacetylases (HDACs) and sirtuins, particularly SIRT2 and SIRT3, have shown delactylation activity in vitro. However, the substrate specificity, cellular targets, and regulation of these enzymes remain unclear. Due to the structural and mass similarity between lactyl and acetyl groups, distinguishing the two poses technical challenges that hinder progress in eraser identification.
As for “readers” (proteins that recognize lactylation marks and mediate downstream effects), no specific domains (like bromodomains for acetylation or chromodomains for methylation) have yet been identified. However, some transcription factors and chromatin remodeling complexes may sense lactylation-induced changes in chromatin states. This area remains in early exploratory stages.
Furthermore, lactylation may compete with or crosstalk with other lysine modifications—such as acetylation, methylation, and ubiquitination—on the same residue. Under stress, hypoxia, or inflammation, these PTMs dynamically interact to shape protein function and gene expression, underscoring lactylation's context- and cell-specific regulatory complexity.
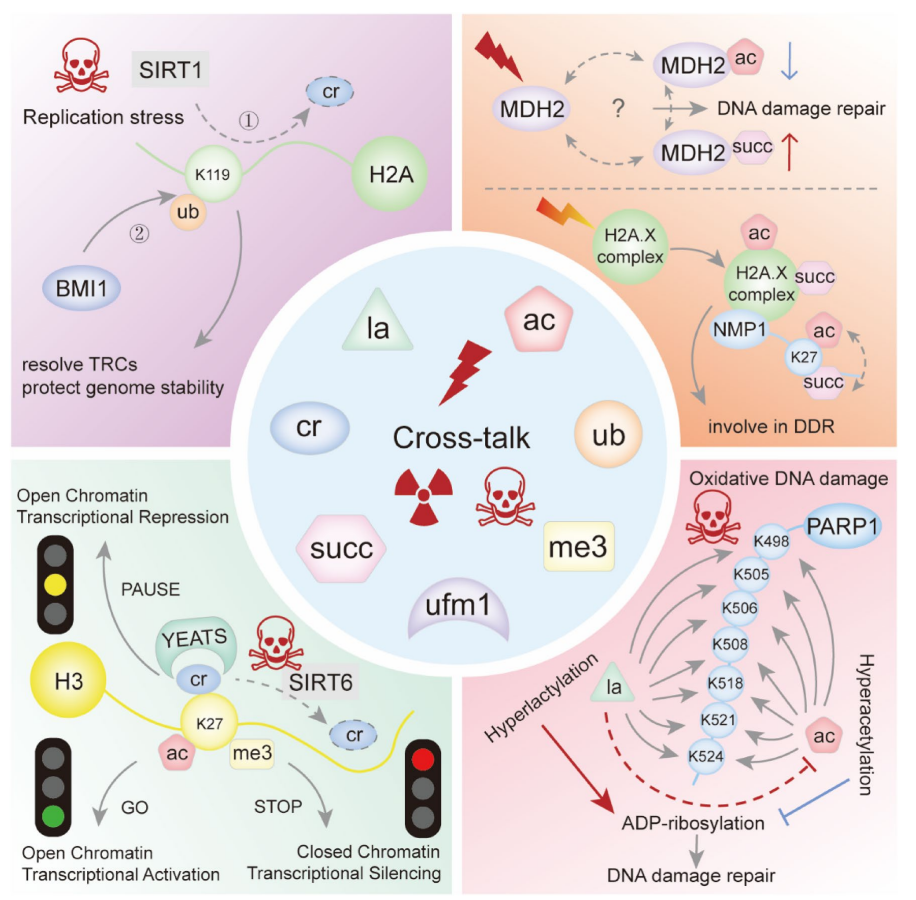
Song, H. et al. Genes Dis. 2022.
Figure 5. Schematic of Cross-talk Involving Lactylation in the DNA Damage Response
Histone and Non-Histone Lactylation
Lactylation was initially discovered on histones, especially histone H3 at lysine residues like H3K18 and H3K9. This modification alters chromatin structure and regulates gene transcription. Elevated histone lactylation levels have been observed under hypoxia and inflammatory stimuli, contributing to the expression of pro-inflammatory cytokines and tissue repair genes.
With the advancement of proteomic technologies, numerous non-histone proteins have also been identified as lactylated. These include transcription factors, metabolic enzymes, and signaling proteins, indicating that lactylation extends beyond chromatin regulation. It plays roles in cellular metabolism, immune surveillance, and cell migration.
Non-histone lactylation typically occurs under metabolic stress (e.g., hypoxia, inflammation). The modification is catalyzed by lactyltransferases, where the lactyl group is covalently attached to lysine. This modification is reversible and alters protein structure, stability, protein–protein interactions, subcellular localization, and enzymatic activity. As such, lactylation contributes to regulation of gene expression, immune responses, cell cycle control, and cell motility.
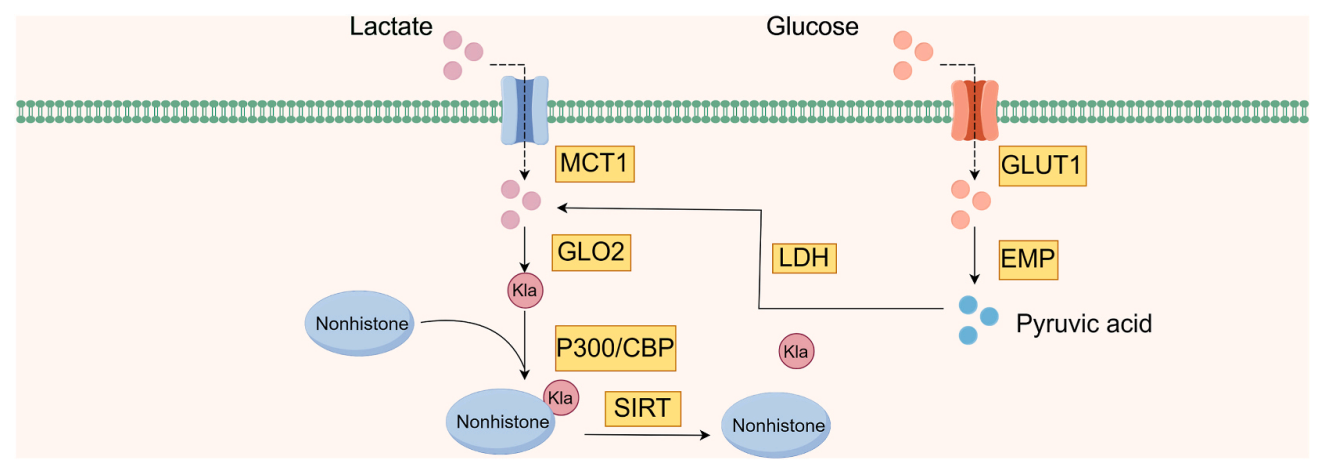
Yu, H. et al. Heliyon. 2024.
Figure 6. Production of Non-Histone Lactylations
Lactylation in Disease: Mechanistic and Pathological Relevance
Protein lactylation is increasingly recognized as a key regulatory mechanism in a range of diseases. At its core, lactylation allows excess intracellular lactate to modify protein function and gene expression, thereby influencing inflammatory, immune, metabolic, and neuronal pathways.
By coupling metabolism and epigenetics, lactylation has been implicated in:
Cancer: promoting tumor cell proliferation, immune evasion, and microenvironmental remodeling.
Cardiovascular diseases: modulating cardiomyocyte metabolism and contractile function.
Neurological disorders: regulating neuronal plasticity and neuroinflammation.
Inflammatory diseases: controlling cytokine expression and immune cell polarization.
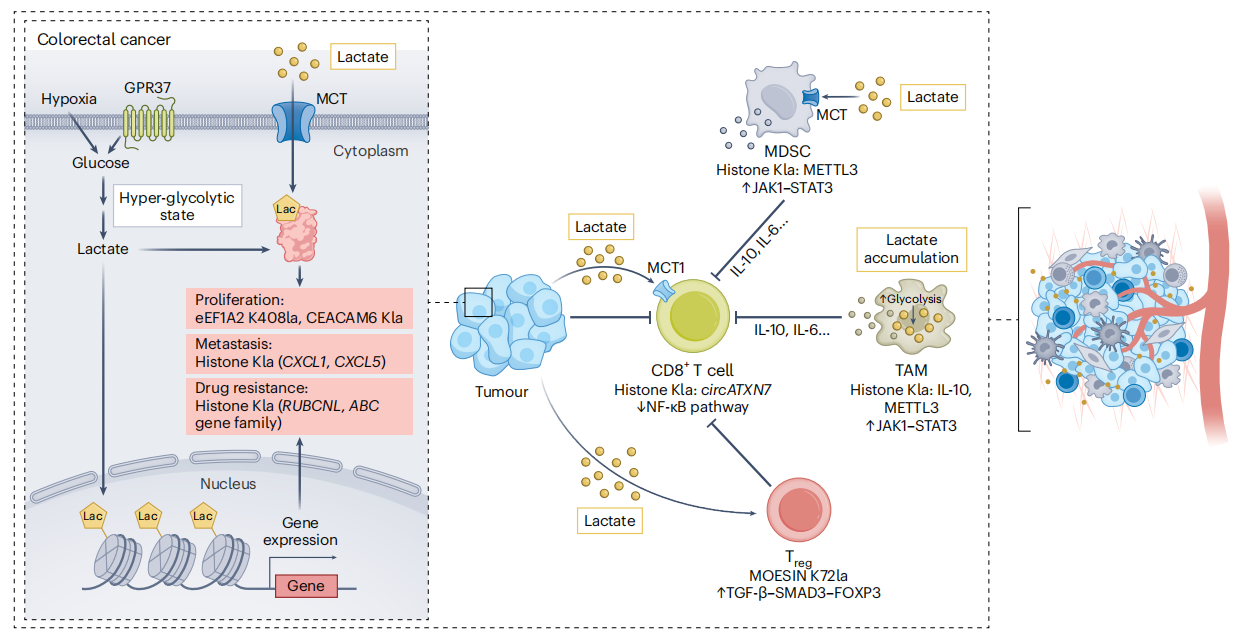
Ren, H. et al. Nat Metab. 2025.
Figure 7. Protein Lactylation in Tumours
Its dynamic, reversible nature makes lactylation a key node in cellular adaptation to environmental stress.
Therapeutic Targets and Intervention Strategies
Given its importance in disease, lactylation is gaining traction as a potential therapeutic target. One strategy is to modulate intracellular lactate levels:
1. Inhibit lactate production using lactate dehydrogenase (LDH) inhibitors.
2. Block lactate export using monocarboxylate transporter (MCT) inhibitors.
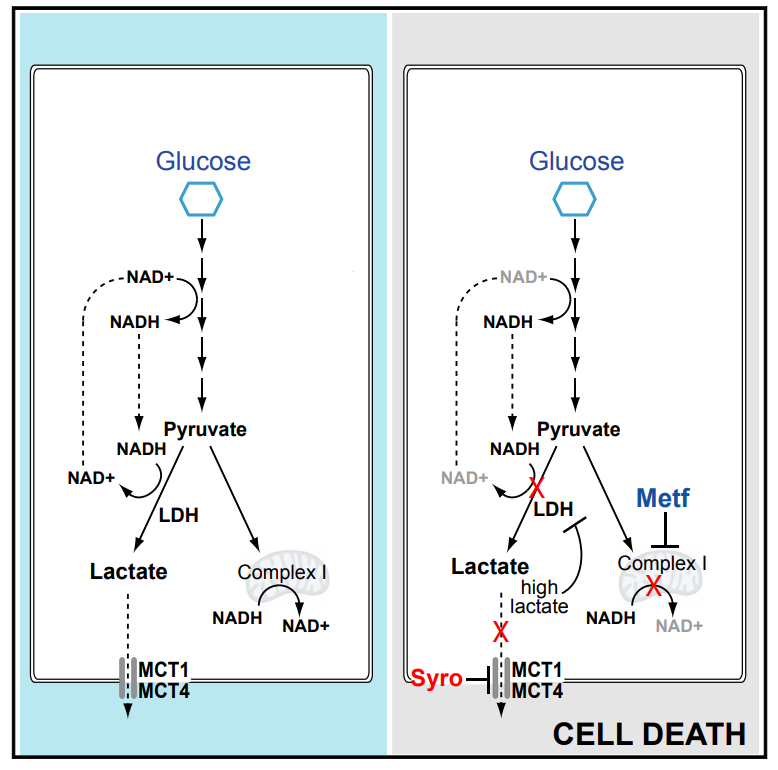
Benjamin, D. et al. Cell Rep. 2018.
Figure 8. The Mechanism of the MCT Inhibitor Syrosingopine in Inhibiting Lactate Production
Parallel efforts are focused on identifying and targeting the enzymes responsible for writing, reading, or erasing lactylation marks. Although no small-molecule drugs specifically targeting lactylation enzymes are in clinical use yet, this research frontier offers great promise in linking metabolic modulation with epigenetic control for precision therapy.
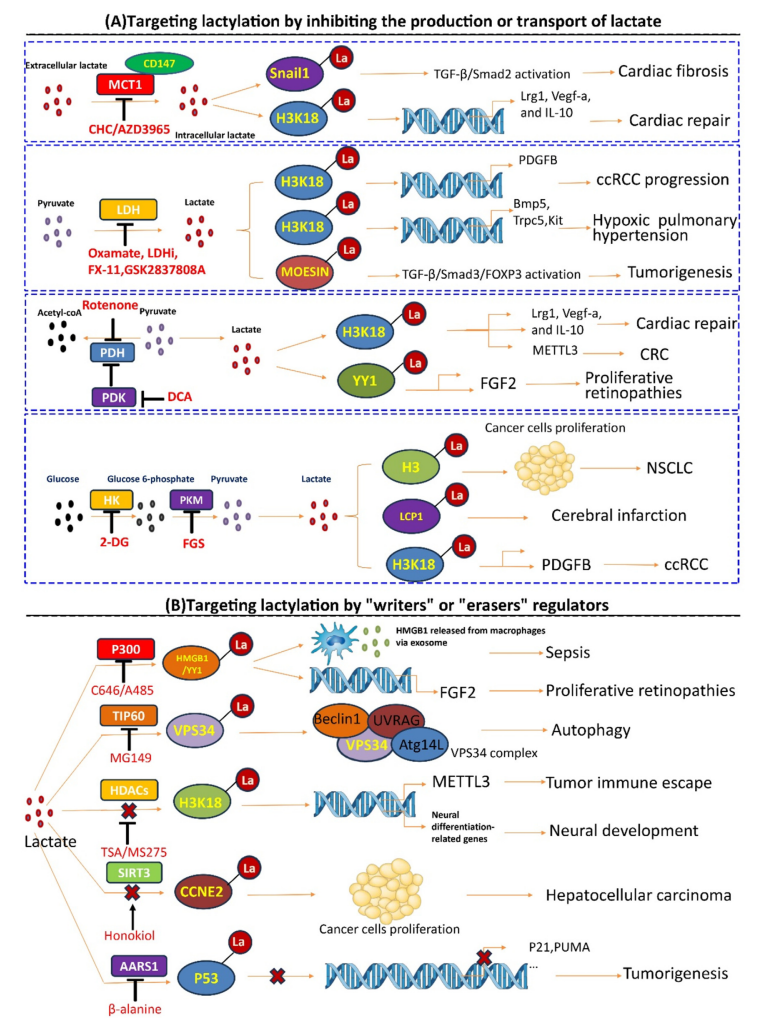
Hu, XT. et al. J Adv Res. 2024.
Figure 9. Targeting Lactylation Regulation Strategy
Analytical Methods for Lactylation
The discovery of protein lactylation has been driven by advancements in mass spectrometry (MS). A typical workflow involves protein digestion, enrichment of lactylated peptides, followed by high-resolution LC-MS/MS analysis. Search engines like pFind and Byonic now support lactylation as a variable modification for peptide identification.
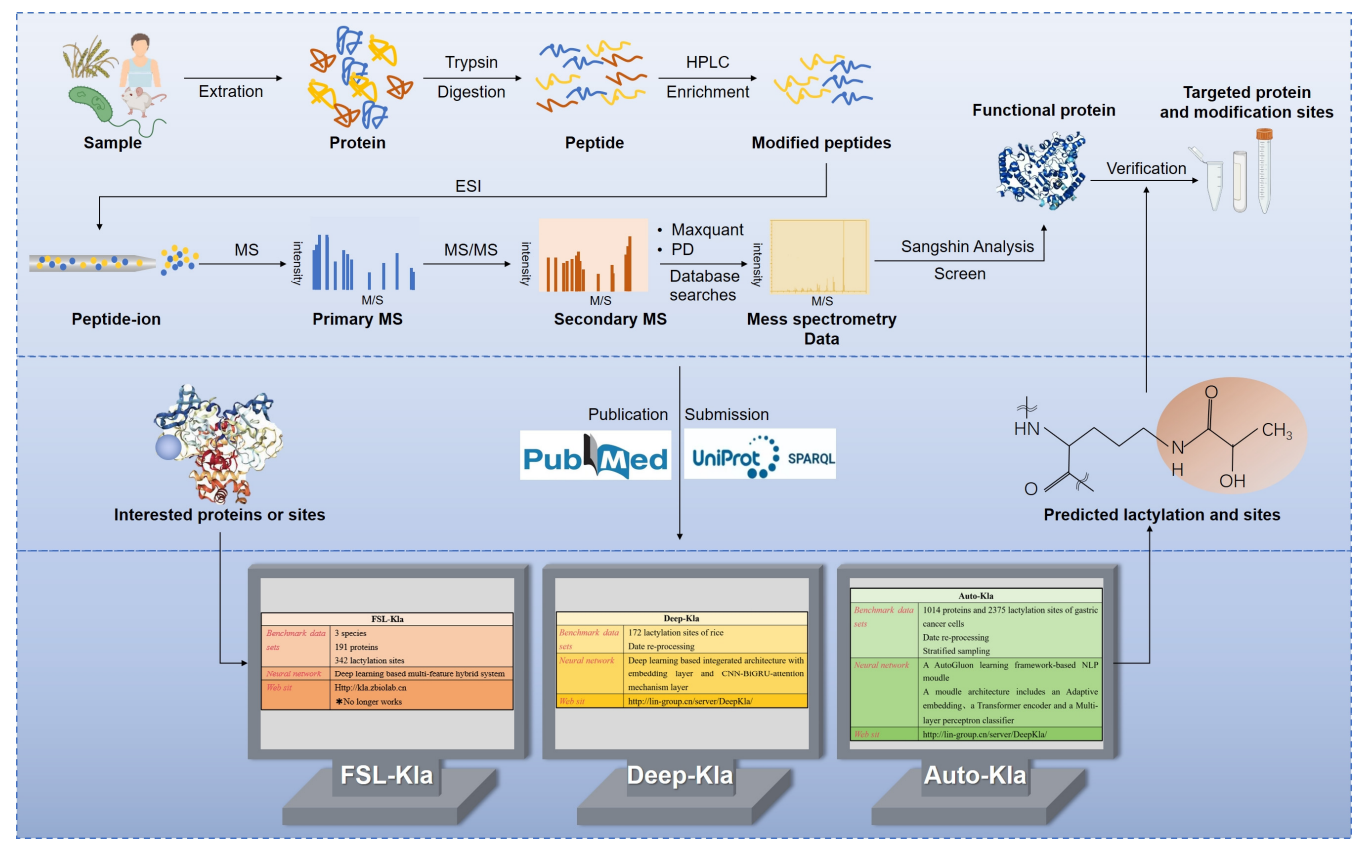
Liu, J. et al. Biomolecules. 2024.
Figure 10. Workflow for the Detection of Lactylation
To improve detection sensitivity and specificity, researchers are developing lactylation-specific antibodies for use in western blotting, immunoprecipitation, and peptide enrichment. As antibody quality improves and multi-modification search algorithms mature, lactylome studies are evolving from discovery to functional and network-level modeling.
Conclusion
Lactylation represents a metabolically driven protein modification that reshapes our understanding of lactate. No longer just a waste product, lactate emerges as a signal that bridges energy metabolism, chromatin regulation, and cellular function. This opens new perspectives for studying disease mechanisms, identifying biomarkers, and developing targeted therapies.
Although the field is still in its infancy—with uncertainties surrounding enzyme specificity and signaling pathways—the scientific and translational value of lactylation is evident. MtoZ Biolabs is committed to advancing proteomics technologies and providing high-quality lactylation analysis services to support fundamental research and translational innovation.
If you are interested in learning more about our lactylation analysis services, please feel free to contact our technical team for customized consultation and project planning.
Related Services
How to order?







