Protein Quality Assessment Service
- Native Proteins: Proteins derived directly from natural sources.
- Recombinant Proteins: Fusion proteins and tagged proteins produced in recombinant systems.
- Antibodies: Various antibody isotypes suitable for detailed characterization.
- Membrane Proteins: Specialized membrane-associated proteins requiring precise handling and analysis.
- Quantity and Concentration: We recommend submitting at least 100 µg of purified protein at a minimum concentration of 1 mg/mL.
- Buffer Conditions: Use minimal detergents or additives; if necessary, please inform us of any specific buffer components included.
- Biopharmaceutical Development: Ensuring therapeutic protein purity and safety.
- Basic Life Science Research: Supporting accurate experimental results with high-quality proteins.
- Structural Biology: Facilitating molecular weight and structural integrity analysis.
- Diagnostic Biomarker Discovery: Improving specificity and sensitivity of biomarkers.
- Bioengineering and Enzymology: Verifying purity and functionality of enzymes in industrial applications.
In the evolving field of life sciences, the integrity and purity of protein samples are paramount for accurate research results and reliable therapeutic applications. MtoZ Biolabs’ Protein Quality Assessment Service is designed to provide researchers and developers with precise, comprehensive evaluations of protein quality. By leveraging advanced mass spectrometry (MS) technologies, we offer a robust assessment framework that delivers detailed insights into protein purity, structural integrity, and consistency, ensuring each sample meets rigorous scientific and industry standards.
Protein quality assessment is fundamental in both basic research and biopharmaceutical development, where high-purity, well-characterized proteins are essential for accurate experimental outcomes and safe therapeutic products. Ensuring that a protein is free from impurities and maintains its intended structure directly impacts its efficacy, safety, and reliability in downstream applications. This process is especially crucial in drug development, where any variance in protein quality can alter therapeutic efficacy or increase the risk of adverse reactions.
Service at MtoZ Biolabs
MtoZ Biolabs, an integrated Chromatography and Mass Spectrometry (MS) Services Provider, provides advanced proteomics, metabolomics, and biopharmaceutical analysis services to researchers in biochemistry, biotechnology, and biopharmaceutical fields. Mass spectrometry has become an indispensable tool for high-sensitivity protein analysis, particularly in evaluating purity, homogeneity, and structural integrity. Unlike traditional methods, MS provides highly specific insights into a protein’s molecular profile, enabling detailed characterization and rapid detection of potential contaminants or modifications.
MtoZ Biolabs is equipped with state-of-the-art mass spectrometry platforms, including the Thermo Fisher Orbitrap Fusion Lumos and other high-resolution MS systems. Through MS, combined with complementary techniques like SDS-PAGE and high-performance liquid chromatography (HPLC), we deliver a multi-faceted protein quality assessment to ensure comprehensive results. These capabilities allow us to detect even minor impurities and variations in the sample, offering clients an unparalleled level of accuracy and reliability.
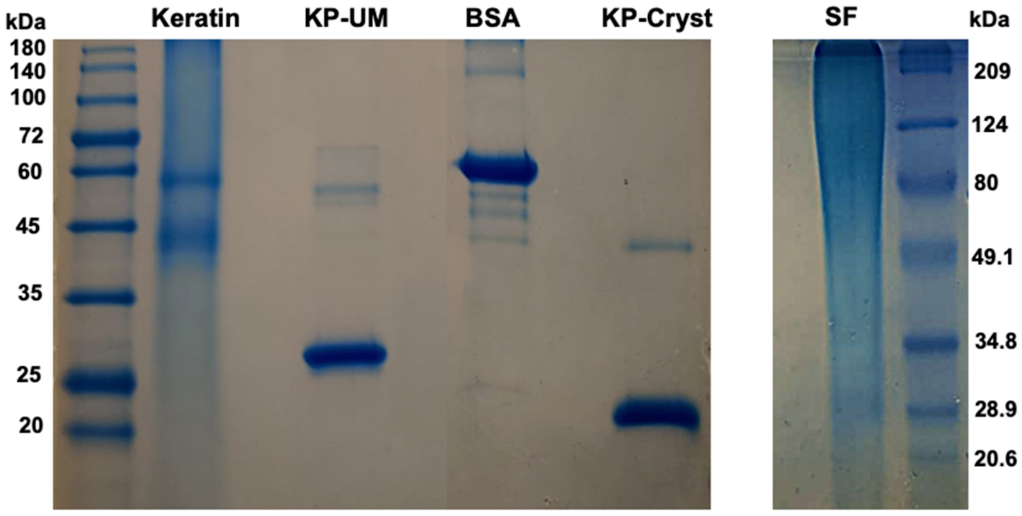
Figure 1. Protein Quality Assessment by SDS-PAGE in a 12.5% Polyacrylamide Gel
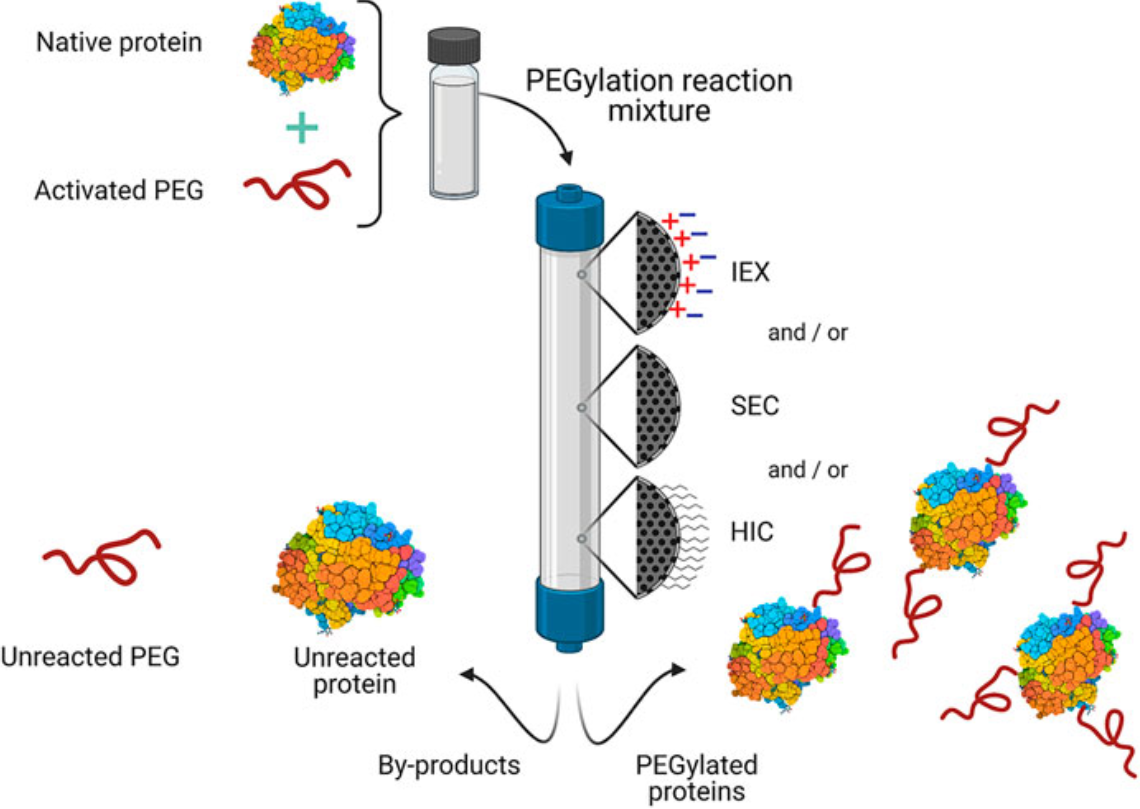
Sánchez-Trasviña, C. et al. Front Bioeng Biotechnol. 2021.
Figure 2. General Scheme for the Purification of Commercial PEGylated Proteins by Chromatography
Service Advantages
1. Advance Analysis Platform: MtoZ Biolabs established an advanced Protein Quality Assessment Service platform, guaranteeing reliable, fast, and highly accurate analysis service.
2. One-Time-Charge: Our pricing is transparent, no hidden fees or additional costs.
3. High-Data-Quality: Deep data coverage with strict data quality control. AI-powered bioinformatics platform integrate all protein quality assessment data providing clients with a comprehensive data report.
4. Customized Reporting: Provides detailed, client-specific reports to support informed research decisions.
5. Rapid Turnaround: Efficient workflows ensure quick and accurate delivery of results.
Sample Submission Suggestions
1. Sample Types
2. Sample Preparation Recommendations
*Note: Please contact us if you have any special requirements or need assistance with your sample preparation.
Applications
Case Study
Purity Comparison of ASNase Products
In a comparative analysis of ASNase products, Onconase and Spectrila were assessed for purity and structural integrity using SDS-PAGE, RP-HPLC, and SEC. In SDS-PAGE (Figures A and B), Spectrila displayed a single sharp band at 36 kDa, representing the ASNase monomer, while Onconase showed multiple additional bands at higher molecular weights, indicating aggregates and impurities. RP-HPLC analysis (Figure C) revealed that Spectrila (black line) exhibited a single sharp peak aligning with the reference standard (gray line), whereas Onconase (red line) showed a broader main peak and several additional peaks. SEC analysis (Figure D) confirmed these findings, with Spectrila (black line) demonstrating minimal aggregation, while Onconase (red line) displayed pronounced aggregate content. The blank sample (blue line) showed no significant signals across all methods. Quantitative analysis showed Spectrila’s purity was nearly 100%, while Onconase contained significant impurities and aggregates, resulting in a purity of only 75%. These results underscore the importance of our Protein Quality Assessment Service in ensuring high-purity protein products, essential for reliable research and therapeutic applications.
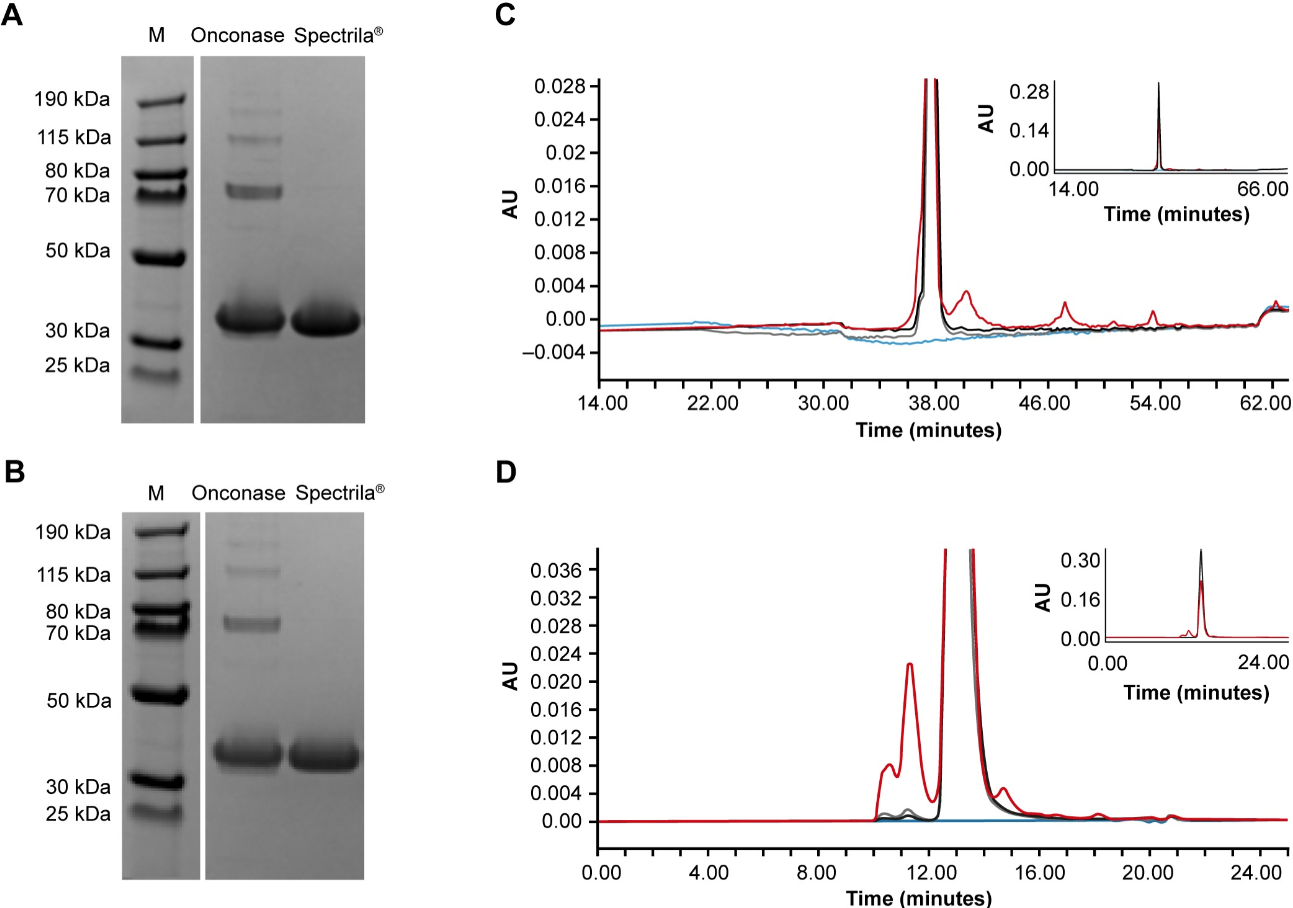
Schnuchel, A. et al. PLoS One. 2023.
Figure 3. Analysis of Protein Purity
Deliverables
1. Comprehensive Experimental Details
2. Materials, Instruments, and Methods
3. Relevant Liquid Chromatography and Mass Spectrometry Parameters
4. The Detailed Information of Protein Quality Assessment
5. Mass Spectrometry Image
6. Raw Data
How to order?







