Purity analysis SEC and RPLC
- Size-Based Separation: SEC separates molecules based on their size as they traverse a porous medium. Larger molecules cannot enter or only partially enter the pores, resulting in faster passage through the column. Smaller molecules can enter more pores, taking longer to elute.
- Physical Separation: It relies on physical size rather than chemical interactions, providing a gentle separation process that does not alter the chemical properties of the molecules.
- Hydrophobicity-Based Separation: RPLC separates molecules based on their hydrophobic interactions with a hydrophobic stationary phase (typically alkyl chain-modified silica, such as C18). Molecules with stronger hydrophobic interactions have longer retention times, while those with weaker interactions elute faster.
- Chemical Separation: The separation is controlled by altering the polarity and composition of the mobile phase, making it suitable for separating molecules with similar structures but different chemical properties.
Size exclusion chromatography (SEC) and reverse phase high-performance liquid chromatography (RPLC) are two highly efficient separation techniques commonly used for the purity analysis of proteins, peptides, and other biological macromolecules.
Technical Principles
SEC
RPLC
MtoZ Biolabs offers professional SEC and RPLC purity analysis services, utilizing advanced chromatography instruments and efficient data analysis tools to provide clients with accurate and reliable sample purity analysis results, supporting scientific research and applications.
Analysis Workflow
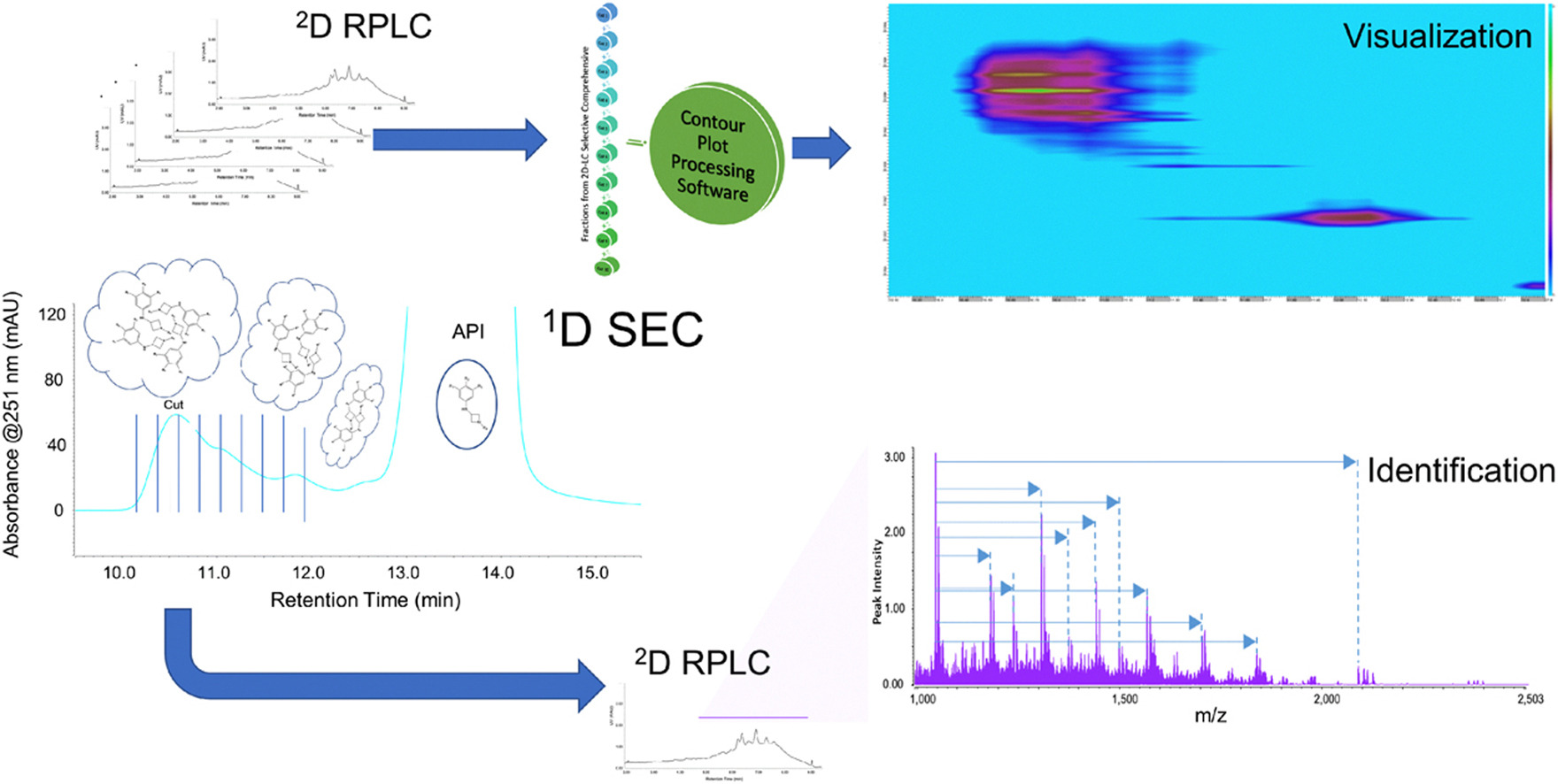
Goyon, A. et al. J Pharm Biomed Anal. 2022.
Sample Submission Requirements
1. Total Conc. of Protein >0.5 μg/L
2. Volume of Protein Sample >50 μL
Please specify the composition of the buffer, if desalt or concentration of protein sample is needed.
Applications
Biopharmaceuticals: Analyze the purity of therapeutic proteins and peptides to ensure product quality and safety.
Protein Research: Evaluate the purity of protein samples to support structural and functional studies.
Quality Control: Conduct quality control during production to ensure the final product meets purity standards.
Impurity Analysis: Detect and quantify trace impurities in samples, optimizing production processes.
How to order?







