Biopharmaceutical Characterization Service
In the realm of modern medical biotechnology, biopharmaceutical development and production entail complex biological systems that utilize proteins and nucleic acids derived from living cells or synthesized via biotechnological methods. These biopharmaceuticals are pivotal in therapeutic and diagnostic applications. Nonetheless, the intrinsic complexity of these medicines can lead to instabilities and impurities during their manufacture, which could compromise drug quality and safety. Accordingly, conducting thorough quality and safety evaluations to ensure compliance with global pharmaceutical regulations is essential before these drugs enter the market.
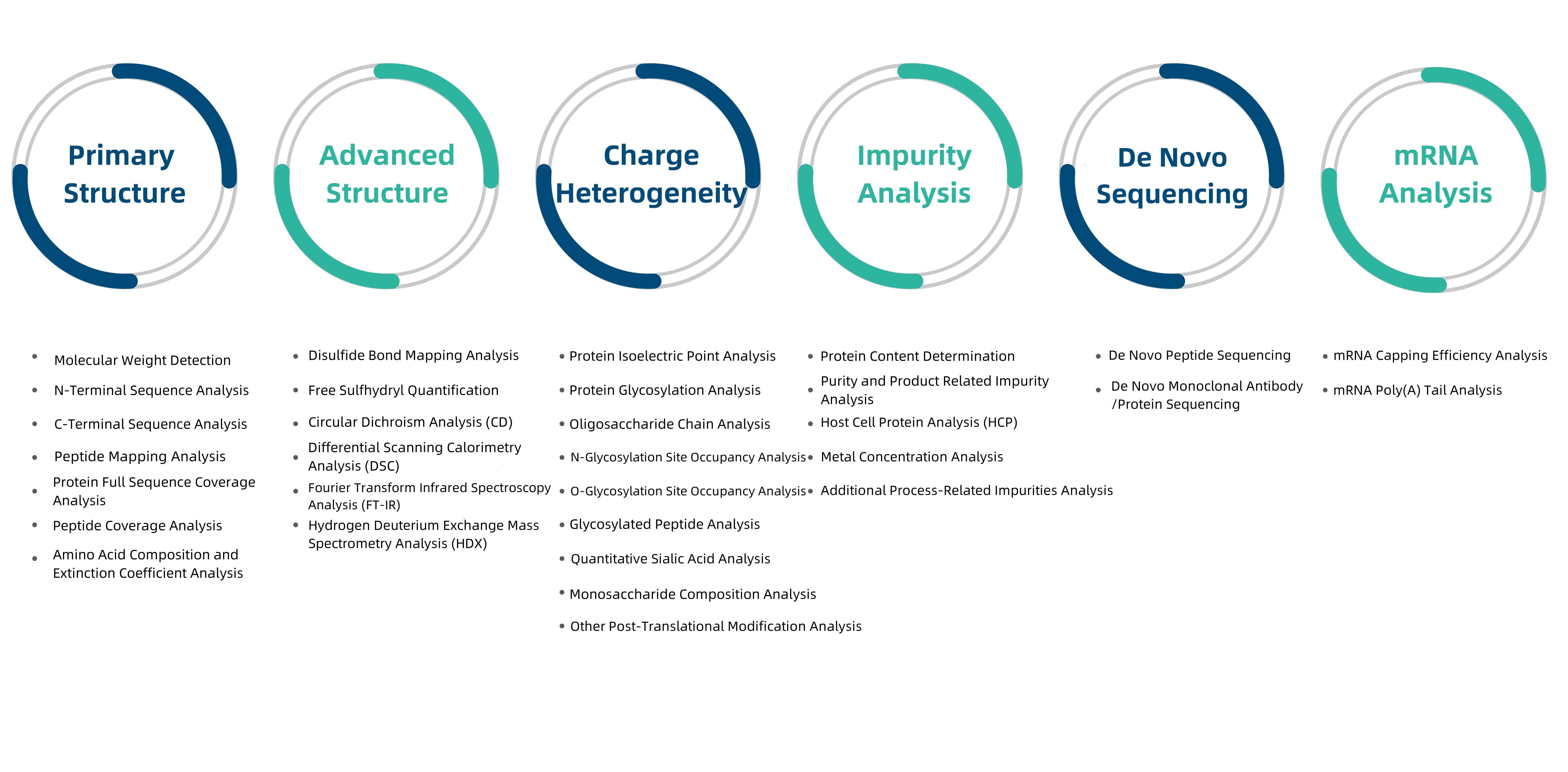
MtoZ Biolabs leverages sophisticated technologies to offer extensive biopharmaceutical characterization services, spanning from the analysis of raw materials to the quality control of the final products. Our principal technologies include mass spectrometry (MS), circular dichroism (CD), crystallography (XRC), and nuclear magnetic resonance (NMR). These technologies form an exhaustive framework for characterization at molecular and sub-molecular levels.
Service at MtoZ Biolabs
Service Advantages
· Team of seasoned experts
· State-of-the-art analytical instruments and methodologies
· Extensive coverage of technological applications
· Expert bioinformatics for data analysis
· Comprehensive client support
Applications
New Drug Development: Verifies that candidate drugs conform to anticipated structural and functional profiles, thereby facilitating the acceleration of their development and refinement.
Production Process Control: Ensures the uniform quality of biopharmaceuticals throughout the manufacturing process, enhancing both efficiency and stability of the final products.
Comparative Quality Analysis: Assists in the market entry and regulatory approval of biosimilars by providing comparative analyses with original pharmaceuticals.
Regulatory Compliance: Offers detailed characterization data that meet international regulatory standards, aiding in the registration and commercialization of drugs.
MtoZ Biolabs is dedicated to delivering comprehensive and accurate biopharmaceutical characterization services. Our efforts aim to expedite the development of pharmaceuticals, mitigate risks associated with research and development, and guarantee successful market introductions. For further details or to engage our services, please contact our team of experts.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Protein Molecular Weight Determination Analysis Service
How to order?







