Biopharmaceutical Identification Analysis Service
Biopharmaceuticals are drugs developed using biotechnological methods, typically derived from microorganisms, plants, or animal cells. Unlike traditional chemically synthesized drugs, the development and production of biopharmaceuticals involve complex biological processes, including genetic engineering, cell culture, and fermentation. These drugs encompass monoclonal antibodies, recombinant proteins, vaccines, gene therapy products, and cell therapy products. Biopharmaceuticals generally have large molecular weights and exhibit high biological activity and specificity. Their three-dimensional structures and functional domains determine their biological activity. Due to their unique biological properties, they are sensitive to environmental conditions such as temperature and pH, necessitating special storage and transportation conditions. Biopharmaceuticals are integral to modern medicine due to their effectiveness in treating complex and severe diseases. According to ICH Q6B guidelines, it is mandatory to ensure the safety and efficacy of biopharmaceuticals before clinical application. However, their complex structures, heterogeneity, and stability present significant challenges for evaluation. With extensive experience and expertise in biopharmaceutical research, MtoZ Biolabs offers comprehensive biopharmaceutical identification and testing services to expedite your biopharmaceutical development process.
Services at MtoZ Biolabs
Biopharmaceutical analysis employs various analytical techniques to assess the quality, safety, and efficacy of biopharmaceutical products. MtoZ Biolabs is equipped with high-performance liquid chromatography (HPLC), mass spectrometry (MS), electrophoresis (SDS-PAGE), and enzyme-linked immunosorbent assay (ELISA). These techniques accurately measure the molecular weight, purity, structure, and content of biopharmaceuticals and evaluate their metabolic processes. These methods are extensively used in drug development, production, and quality control. Additionally, we offer services such as biopharmaceutical N-terminal sequencing, biopharmaceutical N/C-terminal sequencing, and antibody epitope mapping to meet various analytical needs.
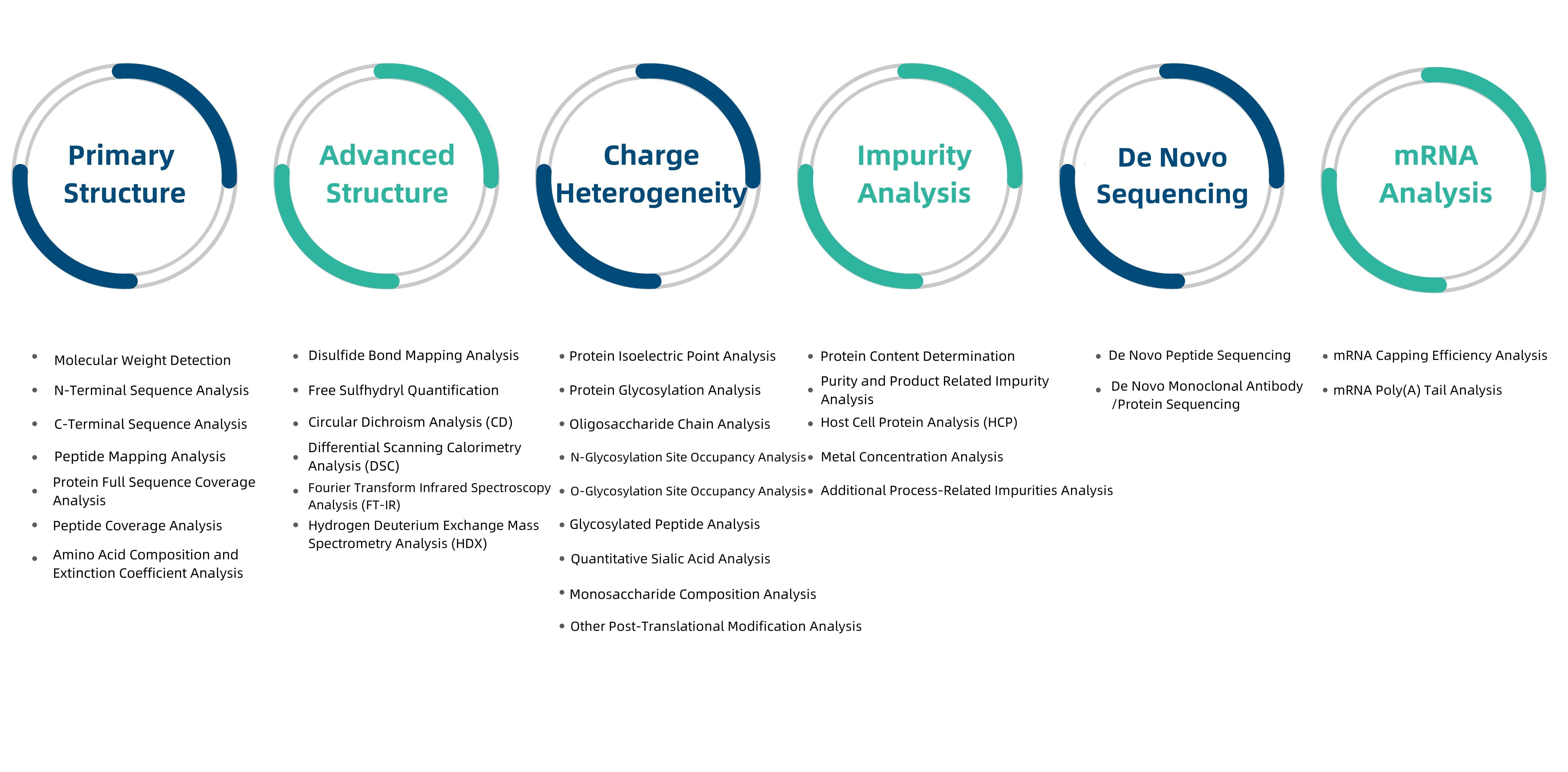
Figure 1. Biopharmaceutical analysis at MtoZ Biolabs
Applications
1. New Drug Development: Screening and optimizing candidate drugs.
2. Process Development: Evaluating and refining production processes.
3. Quality Control: Ensuring product consistency and regulatory compliance.
4. Stability Studies: Assessing the stability of drugs during storage and transportation.
5. Clinical Research: Supporting clinical trial data analysis.
Service Advantages
1. Professional Team: Our experienced technical team ensures the accuracy and reliability of the analysis.
2. Advanced Equipment: We are equipped with state-of-the-art analytical instruments, providing high-precision detection results.
3. Customized Services: We offer tailored analysis solutions based on customer needs.
4. Rapid Response: Our service is characterized by quick sample processing and data analysis, shortening the development timeline.
5. Comprehensive Support: We provide full technical support from sample preparation to data interpretation, ensuring project success.
Biopharmaceutical analysis is critical for ensuring drug quality and safety. It identifies and quantifies drug components, detects potential impurities and degradation products, and evaluates drug stability and efficacy, ensuring the final product meets regulatory standards. MtoZ Biolabs is dedicated to providing high-quality biopharmaceutical analysis services, fostering innovation and development in the biopharmaceutical industry. Free project evaluation, welcome to learn more details! Our technical specialists are available to provide a free business assessment.
Deliverables
1. Experimental Procedures
2. Relevant Mass Spectrometry Parameters
3. Detailed Information on Biopharmaceutical Identification Analysis
4. Mass Spectrometry Images
5. Raw Data
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
Biopharmaceutical N-Terminal Sequencing Service
How to order?







