Comprehensive Peptide Purity Analysis Service
Peptide purity is an essential metric for assessing the concentration of target peptides in synthetic peptide products, critically influencing the outcomes and quality of biomedical research and drug development. The purity of peptides depends on the quality of raw materials, synthesis methods, and post-synthesis treatments. Typical impurities include peptides with missing or truncated sequences, sequences with residual protective groups, and synthesis byproducts.
At MtoZ Biolabs, we utilize the Orbitrap Fusion Lumos mass spectrometry platform by Thermo Fisher, paired with nano-liquid chromatography, to deliver peptide purity analysis services. This platform enables precise detection and quantitative assessment of impurities in peptide samples. We further enhance our analysis by incorporating ultraviolet light detection (UV detection), providing a spectrum of services from basic purity tests to comprehensive impurity profiling.
Analysis Workflow
1. Sample Preparation
Treat peptide samples by dissolving and filtering to prepare them for analysis.
2. HPLC Analysis
Employ advanced RP-HPLC techniques to determine peptide purity through chromatographic quantification, and assess potential byproducts qualitatively and quantitatively.
3. MS Analysis
Use electrospray ionization (ESI MS) and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF MS) mass spectrometry for accurate determination of peptide molecular weights and structural confirmation.
4. Data Analysis and Report Generation
Utilize sophisticated software for analyzing data from HPLC and MS, producing detailed analytical reports.
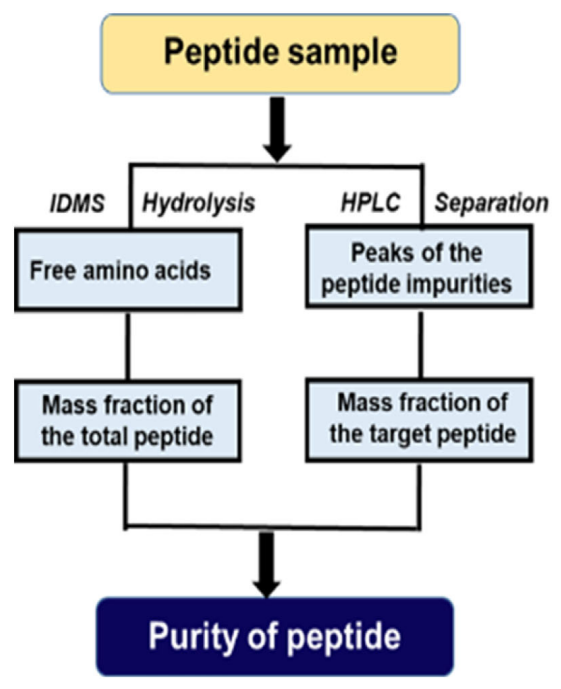
Lee, H. et al.Bull Korean Chem Soc.2022.
Figure 1. Analysis Workflow of Peptide Purity
Service Advantages
High Precision: By integrating HPLC and MS technologies, we achieve highly sensitive detection of minute impurities.
Swift Turnaround: We optimize our workflow from sample reception to report production to guarantee rapid results delivery.
Expert Analysis: Data are interpreted by our experienced analysts, offering specialized technical support and consultancy.
Applications
Guaranteeing the safety and effectiveness of pharmaceuticals
Improving the functionality and stability of biomarkers
Supporting preclinical studies to validate drug structures
Maintaining rigorous quality control during manufacturing
Deliverables
1. Experimental Procedures
2. Relevant Mass Spectrometry Parameters
3. Detailed Information on Peptide Purity
4. Mass Spectrometry Images
5. Raw Data
* For research use only. Not for diagnostic purposes. Samples from individual customers or for personal use are not accepted.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
RP-HPLC Peptide Purity Analysis Service
MS Peptide Purity Analysis Service
How to order?







