IgM Antibody Sequencing Service
Antibodies are proteins synthesized by B cells, functioning to identify and neutralize foreign substances (antigens). Antibodies are categorized into five major subtypes based on their structural and functional characteristics: IgG, IgA, IgM, IgE, and IgD. IgM is the earliest antibody to appear during an immune response, predominantly secreted as pentamers in the bloodstream, accounting for roughly 5% to 10% of the total serum antibodies. Despite its relatively modest neutralizing capacity, the early presence of IgM serves as a crucial marker for the early diagnosis of infections. Therefore, sequencing IgM antibodies enhances diagnostic accuracy, offers deeper insights into their molecular mechanisms in immune responses, and provides a scientific foundation for vaccine development and disease treatment.
MtoZ Biolabs leverages the high-resolution Orbitrap Fusion Lumos mass spectrometer and a wealth of bioinformatics expertise to offer a pioneering antibody sequencing platform. This platform adeptly analyzes various antibody subtypes (such as IgM and IgG) and formats (including fluorescently labeled, immobilized, and multispecies antibodies), providing one-stop services that ensure comprehensive coverage of antibody sequences and detailed reports.
Analysis Workflow
1. Sample Preparation
Collect biological samples containing IgM antibodies.
2. Antibody Extraction and Purification
Employ specific affinity chromatography techniques to isolate and purify IgM antibodies.
3. Enzymatic Digestion
Process the antibodies with enzymes to generate suitable fragments for MS.
4. LC-MS/MS Analysis
Conduct separation of antibody fragments via LC followed by precise measurement using LC-MS/MS.
5. Data Analysis
Analyze the MS data with advanced bioinformatics tools to determine the antibody sequences.
Service Advantages
1. High Sensitivity and Accuracy
Capable of accurately detecting and analyzing IgM antibody sequences in trace samples.
2. Comprehensive Coverage
Provides complete IgM antibody sequence information, including all critical peptide segments.
3. Rapid Efficiency
Streamlines the entire workflow from sample reception to final report, significantly reducing research time.
4. Versatility
Not only sequences IgM antibodies but also analyzes their post-translational modifications (PTMs).
Sample Submission Requirements
1. Sample Types: Serum, tissue samples, purified proteins, etc.
2. Sample Volume: A minimum of 1-2 mL of serum or an equivalent amount of other samples.
3. Sample Preservation: Samples should be transported and stored at low temperatures, preferably using dry ice or liquid nitrogen.
Applications
1. Vaccine Development
Supports the application of IgM antibodies in vaccine research and development.
2. Disease Diagnosis
Facilitates the diagnosis and monitoring of IgM antibody-related diseases.
3. Immunology Research
Explores the functions and mechanisms of IgM antibodies in basic immunological research.
4. Biopharmaceuticals
Assists in the development of IgM antibody-based biopharmaceuticals.
Sample Results
In-Depth Analysis of Human Neonatal and Adult IgM Antibody Repertoires
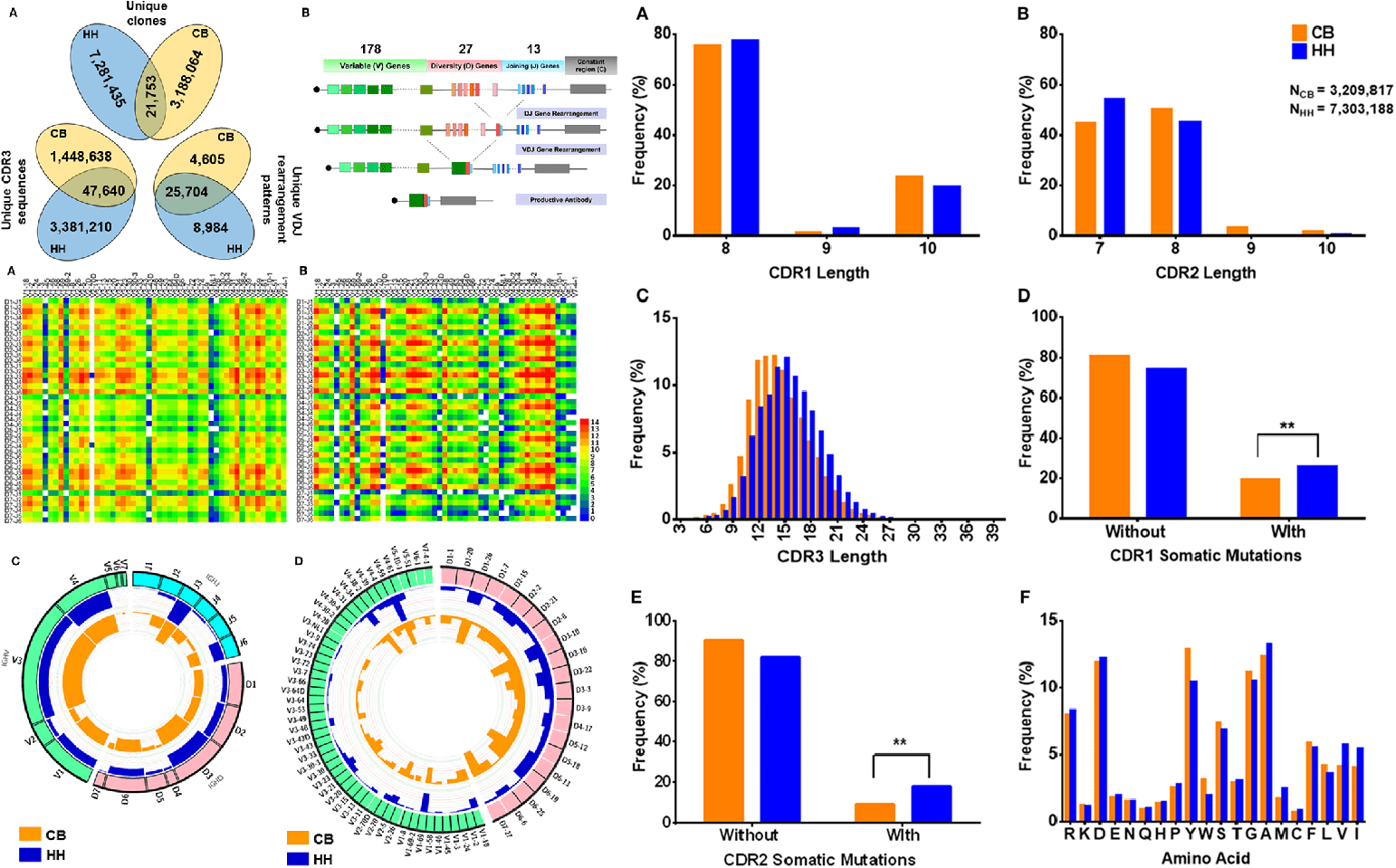
Hong, B. B. Front. Immunol. 2018.
IgM Antibodies Against Malondialdehyde and Phosphorylcholine in Different Systemic Rheumatic Diseases
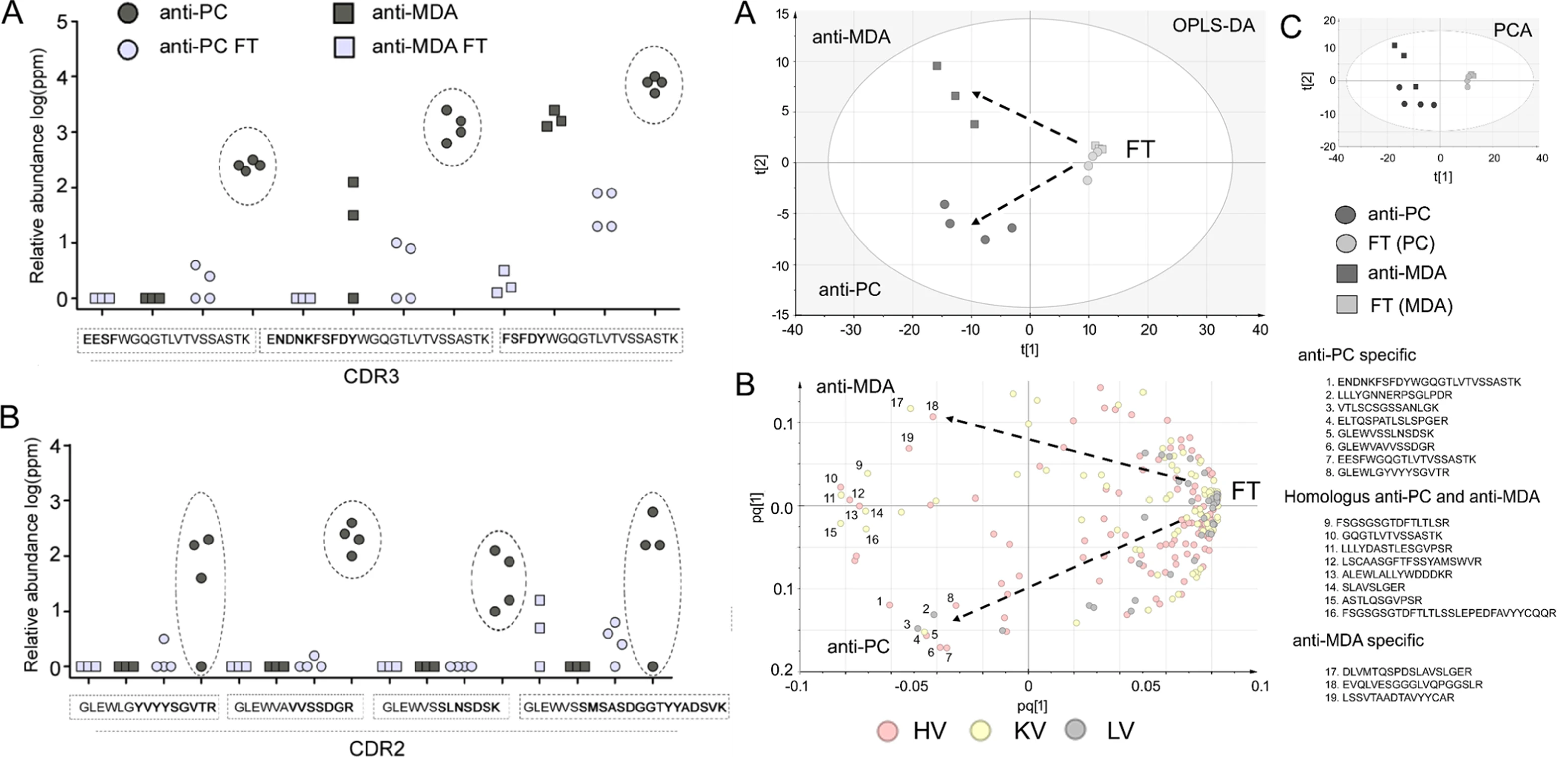
Divya, T. Sci. Rep. 2020.
Deliverables
1. Experimental Procedures
2. Relevant Mass Spectrometry Parameters
3. Detailed Information on IgM Antibody Sequence Analysis
4. Mass Spectrometry Images
5. Raw Data
FAQ
Q1: What should be done if IgM antibodies degrade during sample preparation?
To prevent degradation of IgM antibodies, it is essential to handle samples under strictly controlled conditions. Always keep samples refrigerated and add specific protease inhibitors to the sample buffer. Additionally, minimize the time from sample collection to processing to reduce degradation.
MtoZ Biolabs, an integrated chromatography and mass spectrometry (MS) services provider.
Related Services
IgA Antibody Sequencing Service
How to order?







