Glycosylation Site Detection Service
- Comply with ICH Q6B guidelines to ensure biopharmaceutical samples meet new market standards.
- Analyze glycosylation profiles across a variety of proteins, antibodies, and cells.
- Identify and study glycan biomarkers.
- Compare glycosylation patterns across different samples.
- Detect and characterize unusual glycosylation events.
- Maintain batch-to-batch consistency during production.
Glycosylation, especially N-glycosylation, is a universal post-translational modification in regarding the localization, function, activity of proteins in tissues and cells. The study of the N-glycosylation site is an important prerequisite for understanding the function of sugar chain of the target protein. Among the information related to N-glycosylation, the site of N- glycosylation is the basis for our understanding of its sugar chain function. Besides the application of point mutation in traditional biochemistry, the study of glycosylation sites has also played an important role in the identification of glycosylation sites in N-terminal. MALDI TOF MS and nano LC-ESI-MS/MS are commonly used to identify the N/O-glycosylation sites in the antibodies.
MtoZ Biolabs uses both MALDI TOF MS and nano LC-ESI-MS/MS technology to provide efficient and accurate Glycosylation Site Detection of multiple biopharmaceuticals, including proteins, antibodies, etc. Upon the sample reception, Fc domain and Fab of the antibodies are first separated, followed by digestion into peptide fragments, and analysis of glycosylation site by MALDI TOF MS and nano LC-ESI-MS/MS.
Analysis Workflow
1. Digestion of Biopharmaceutical Samples by Trypsin or Other Appropriate Proteases
2. Separation of N-Glycopeptides and O-Glycopeptides Through Chromatographic Column
3. Deglycosylation Treatment
4. Peptide Analysis by MALDI-TOF MS, or ESI-MS/MS
5. Analysis of the N/O-Glycosylation Site Based on the Mass Spectra Results
6. Comparison of the Analytical Results of Glycosylation Sites of Different Batch Samples
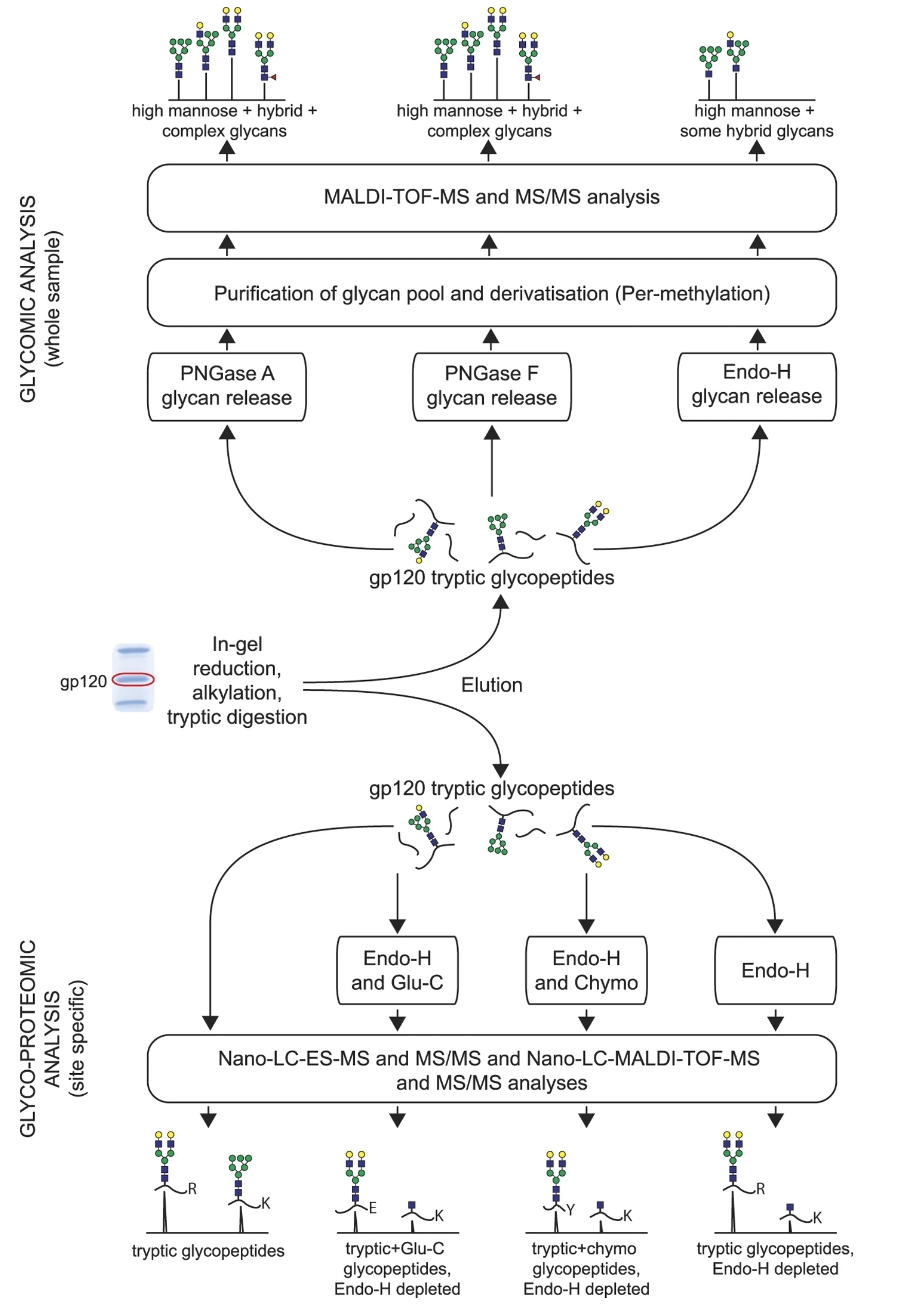
Panico, M. et al. Sci. Rep. 2016.
Figure 1. Glycosylation Site Analysis of Antibody
Service Advantages
1. High Sensitivity
Detects even low-abundance glycosylation sites.
2. High Accuracy
Ensures precise localization of glycosylation sites and a thorough analysis of glycan structures.
3. Customized Services
Tailored analysis plans to meet specific customer requirements.
Sample Submission Requirements
The types of samples we accept include therapeutic antibodies/recombinant proteins, serum/plasma, cell culture media, cell lines, and more. It is recommended that each sample contains at least 500 µg of total protein. For low-abundance samples, a larger sample quantity may be necessary to ensure reliable results.
For more sample details, please consult our technical team.
Applications
Deliverables
1. Experiment Procedures
2. Parameters of Liquid Chromatography and Mass Spectrometer
3. MS Raw Data Files
4. Glycosylation Site Results
5. Bioinformatics Analysis
Related Services
Identification of Biopharm
Variation Analysis
Purity Analysis
How to order?







