Resources
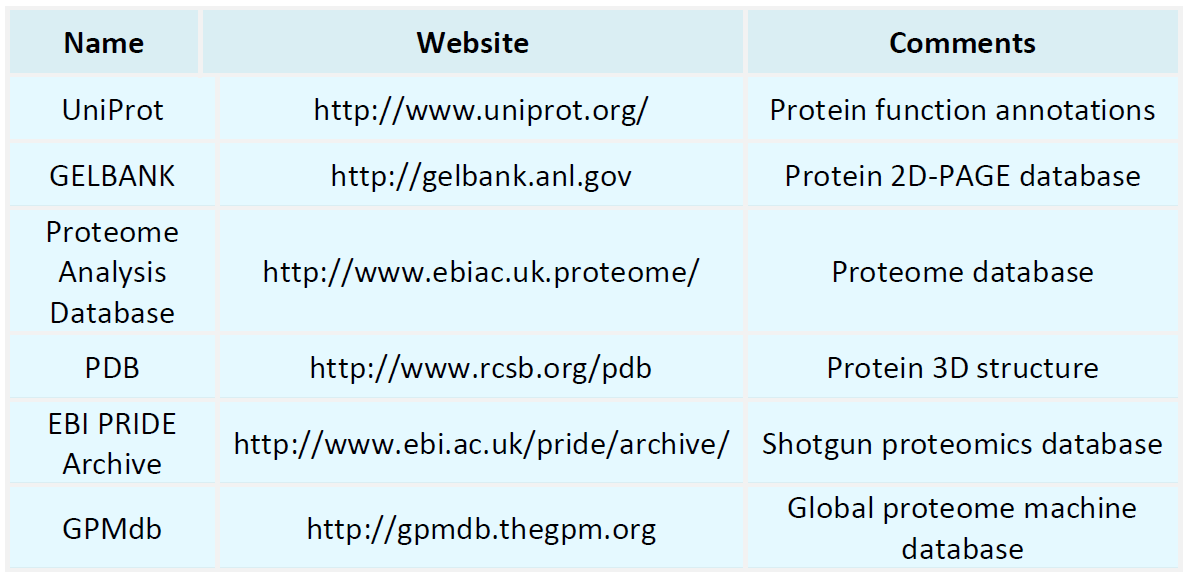
Proteomics Databases
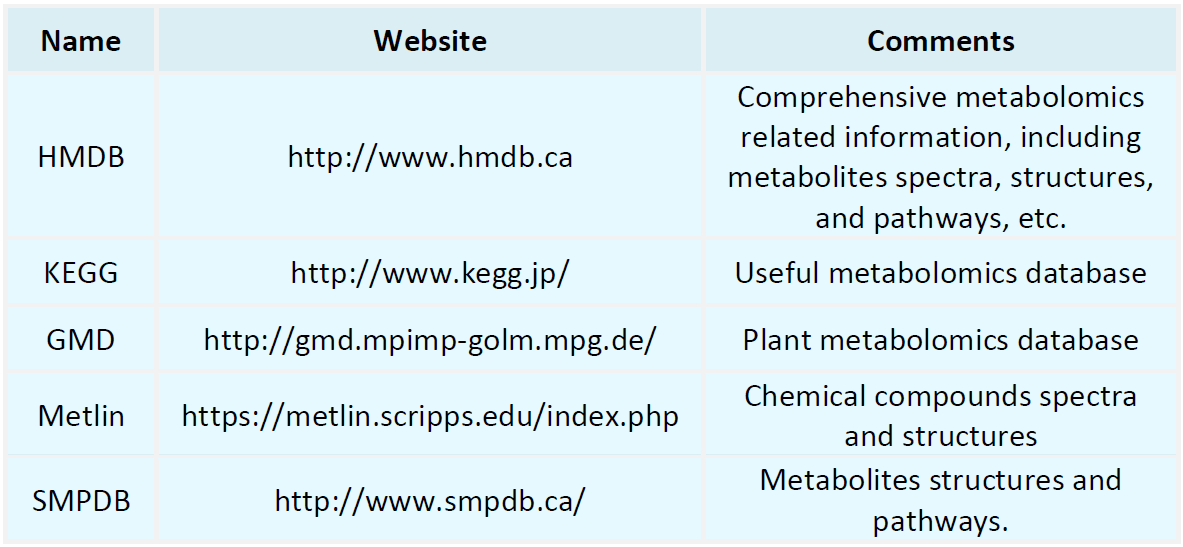
Metabolomics Databases
-
• Protein Coverage Mass Spectrometry
Protein coverage mass spectrometry is a high-resolution analytical method utilized to assess the coverage of protein amino acid sequences, extensively used in proteomics research and biopharmaceutical development. This technique enables the precise analysis of protein peptide fragments via mass spectrometry, producing coverage maps to verify whether a protein's complete or partial sequence is detected. The extent of protein coverage is indicative of the analysis's depth and precision, crucial for eluc......
-
• Protein Concentration Analysis
Protein concentration analysis is a technique aimed at determining the protein content in biological samples, with widespread applications in life sciences research and biopharmaceutical development. Proteins, as fundamental components of biomolecules, are central to cellular functions, signal transduction, and metabolic regulation. Accurate protein concentration measurement is essential not only for basic biological experiments but also for applications such as disease diagnosis, drug development, an......
-
• Advances in Protein Content Analysis Methods
Protein content analysis is a critical technique for quantitatively measuring the total amount of protein in a sample, and it is fundamental to life sciences research and industrial applications. As key mediators of biological functions, proteins maintain cellular processes, regulate physiological activities, and enable adaptation to environmental changes. Analyzing protein content allows researchers to obtain essential biological information about a sample, supporting subsequent functional studies, d......
-
Protein complex analysis is a technique used to study the interactions between proteins and their compositions. By revealing the structure, function, and dynamic changes of protein complexes, it helps scientists understand the complex biological processes occurring within organisms. A protein complex refers to a functional unit formed by two or more proteins through specific physical and chemical interactions. These complexes perform a variety of biological functions within the cell, such as signal tr......
-
• Protein Composition Analysis
Protein composition analysis is a crucial methodology for investigating the variety, abundance, and dynamic alterations of proteins in biological samples. By elucidating the complex network and functional mechanisms of proteins, it drives forward both fundamental research and applied developments in life sciences. As essential components of living organisms, proteins participate in nearly all biological processes. This analysis assists scientists in understanding cellular molecular changes under diffe......
-
• Plant Protein Complete Amino Acid Profile
Plant protein complete amino acid profile is a key research area for assessing the nutritional value and functional characteristics of plant proteins. With advantages such as abundant sources and environmental sustainability, plant proteins have become a popular choice for human dietary protein and industrial applications. Amino acids are the basic building blocks of proteins, and their types and proportions directly determine the quality, functionality, and potential applications of plant proteins. C......
-
• Peptide Fragment Fingerprinting
Peptide fragment fingerprinting (PFF) is a crucial tool for analyzing protein structures and identifying proteomes. Utilizing mass spectrometry, this technique analyzes peptides derived from protein hydrolysis to produce distinctive mass spectra. Each protein generates a unique peptide fragment fingerprint during mass spectrometry analysis, akin to a personal fingerprint. This distinctiveness makes PFF an essential method for protein identification and quantification in proteomic research. PFF plays a......
-
• Western Blot Band Quantification
Western blot band quantification is one of the key techniques in protein research, primarily used to detect the expression levels of specific proteins and to perform relative or absolute quantification based on the grayscale intensity of bands. Western blot (WB) technology is based on protein electrophoretic separation, antibody-specific recognition, and chemical or fluorescent signal detection, enabling the precise identification of target proteins within complex samples. Compared to traditional West......
-
• N-Terminal Sequencing in Protein Analysis
N-terminal sequencing, the process of determining an amino acid sequence starting from the N-terminal (amino-terminal) of a protein, uses chemical or enzymatic degradation methods to identify the sequence of amino acids in a protein or polypeptide chain. This technique is widely used in biological research. In the discovery and identification of novel proteins, N-terminal sequencing provides direct information on amino acid sequences, which assists in predicting protein function and structure. Underst......
-
• Parameters Required to Characterize a Protein
Proteins are fundamental to biological processes in living organisms and serve as the primary components of biological organs and tissues. Protein characterization, which involves obtaining specific structural and functional information, is a critical step in biological research. A comprehensive characterization of a protein typically requires the determination of the following parameters: sequence information, molecular weight, secondary structure, tertiary structure, quaternary structure, hydrophobicity..
How to order?







