Resources
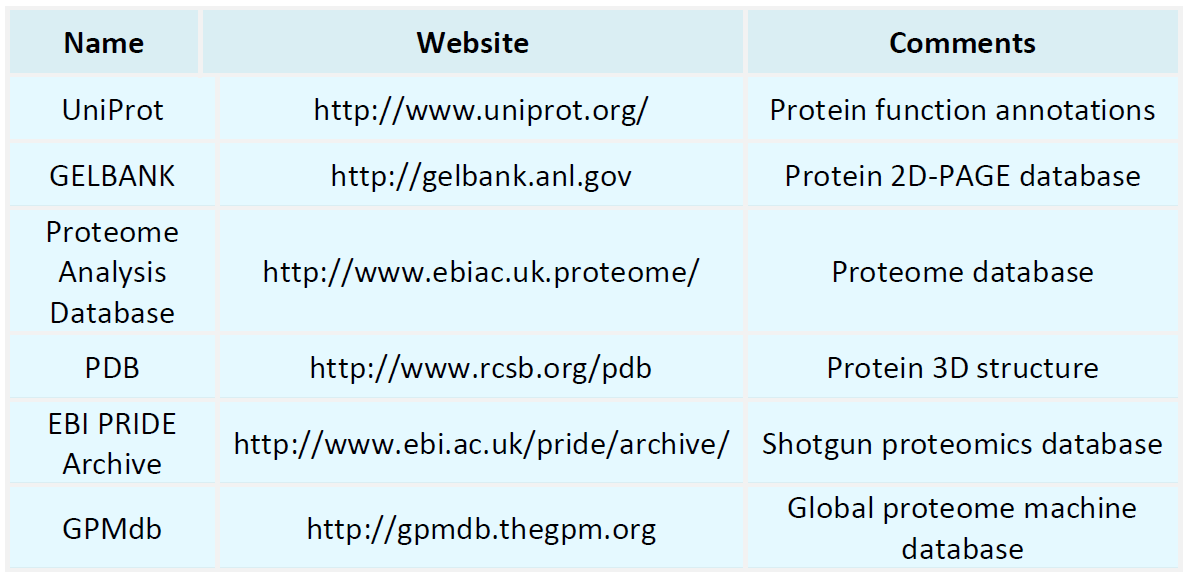
Proteomics Databases
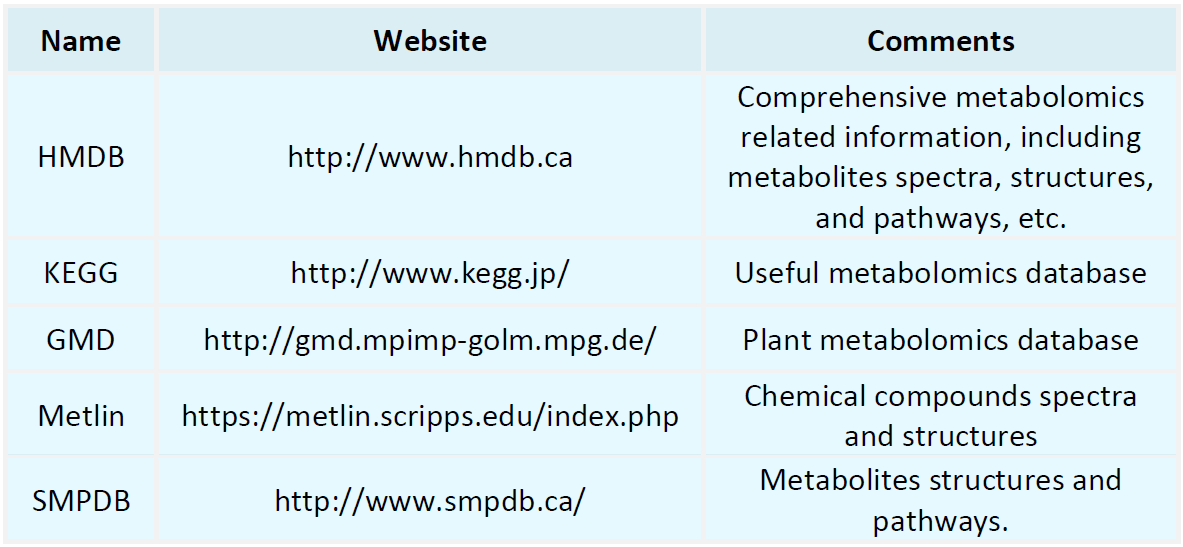
Metabolomics Databases
-
• Deglycosylation in Protein Mass Spectrometry
Protein mass spectrometry is an essential technique in biotechnology for analyzing protein composition, structure, and properties. In protein mass spectrometry, deglycosylation is commonly used to remove glycan moieties from proteins, allowing determination of their intact molecular weight. Mass spectrometry analysis following deglycosylation provides key insights, including the intact molecular weight of the protein, the glycosylation sites, and structural information.
-
• What Purity Is Required for the Identification of Polypeptide Secondary Structure
Peptide secondary structure identification relies on sample purity as a key parameter, as the degree of purity directly influences the accuracy and reliability of experimental outcomes. Purity Requirements: To achieve accurate and reproducible results, peptide samples should have a minimum purity of 90%. The presence of significant impurities in a sample can interfere with experimental outcomes, leading to deviations in identification accuracy. In certain cases, particularly for structurally complex.......
-
• Preparation of Single Cell Suspensions for Mass Cytometry
Materials and Instruments: 1. Experimental cell lines 2. Phosphate-buffered saline (PBS) 3. 0.25% Trypsin-EDTA 4. Centrifuge 5. Flow cytometer Preparation Procedure: 1. Cell Washing and Harvesting: Begin by washing the cells with PBS. Following this, treat the cells with 0.25% Trypsin-EDTA to facilitate cell detachment and harvesting.
-
• Requirements for Samples in Protein Immunoprecipitation-Mass Spectrometry
Immunoprecipitation-Mass Spectrometry (IP-MS) is a robust technique for analyzing protein-protein interactions. Achieving reliable and reproducible results hinges on the quality and handling of the samples. The following outlines the sample requirements for IP-MS concerning "purity, integrity, quantity, buffer, and antibody selection." 1. Sample Purity: Samples need to be adequately pure to minimize nonspecific protein interactions. Prior to IP-MS, samples should be subjected to thorough washing and........
-
• Conditions for Protein Mass Spectrometry Quantification
Protein mass spectrometry quantification is a crucial analytical approach for investigating protein structures and functions. This method synthesizes theoretical knowledge, experimental techniques, instrumentation, data processing, and experimental design with result interpretation, thereby providing robust tools and methodologies for comprehensive protein research.
-
• Can Mass Spectrometry Measure Large Proteins
Mass spectrometry (MS) is a powerful analytical technique that enables precise mass measurement and structural characterization of biomolecules. In biomedical research, MS has become an indispensable tool for the identification and quantification of proteins and other macromolecules. Principles and Methods: MS operates based on the mass-to-charge ratio (m/z) of ionized molecules. The process involves ionizing the sample, accelerating the resulting ions in an electric or magnetic field, and measuring their..
-
• Calculation of m/z for Polypeptides in Mass Spectrometry
Calculation of m/z for Peptides in Mass Spectrometry: In mass spectrometric analysis, the mass-to-charge ratio (m/z) is a fundamental parameter for identifying ions in a spectrum. Each peak represents an ion, and its m/z value is derived by dividing the mass of the ion by its charge. Typically, the charge (z) is +1 or -1, as most ions are singly charged. However, larger molecules, such as proteins and peptides, may exhibit multiple charges, significantly affecting their ionization.
-
• Protein Analysis Using Liquid/Gas Chromatography-Mass Spectrometry
Mass spectrometry is a powerful analytical technique used to determine the mass, chemical structure, and purity of a sample. In biological research, it is widely employed for the identification of proteins and other macromolecules. By providing detailed structural and compositional information, mass spectrometry plays a crucial role in advancing our understanding of these complex biomolecules.
-
• Naming of Gene Knockout Cell Lines
In molecular biology, gene knockout is an experimental technique used to disable the function of a specific gene in an organism through genetic engineering. This method is valuable for investigating the roles of particular genes and their potential links to diseases. In cell culture, gene knockout cell lines are created by genetically engineering cells to inactivate specific genes. These cell lines serve as powerful tools for studying disease mechanisms, drug screening, and understanding gene functions.
-
• Immunoprecipitation Mass Spectrometry Protocol
Immunoprecipitation Mass Spectrometry (IP-MS) is a widely used technique for identifying protein-protein interactions, integrating immunoprecipitation with mass spectrometry-based proteomic analysis. 1. Sample Preparation: Protein extraction is performed from cells or tissues by lysing the samples with an appropriate lysis buffer, such as radioimmunoprecipitation assay (RIPA) buffer, followed by centrifugation to remove cell debris and isolate soluble proteins.
How to order?







