Resources
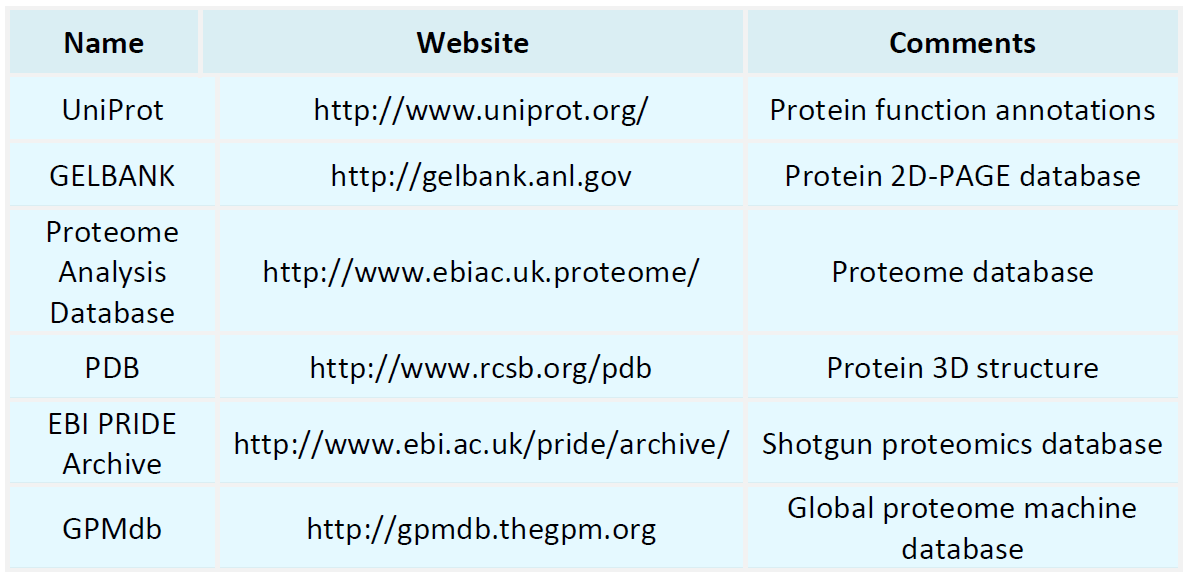
Proteomics Databases
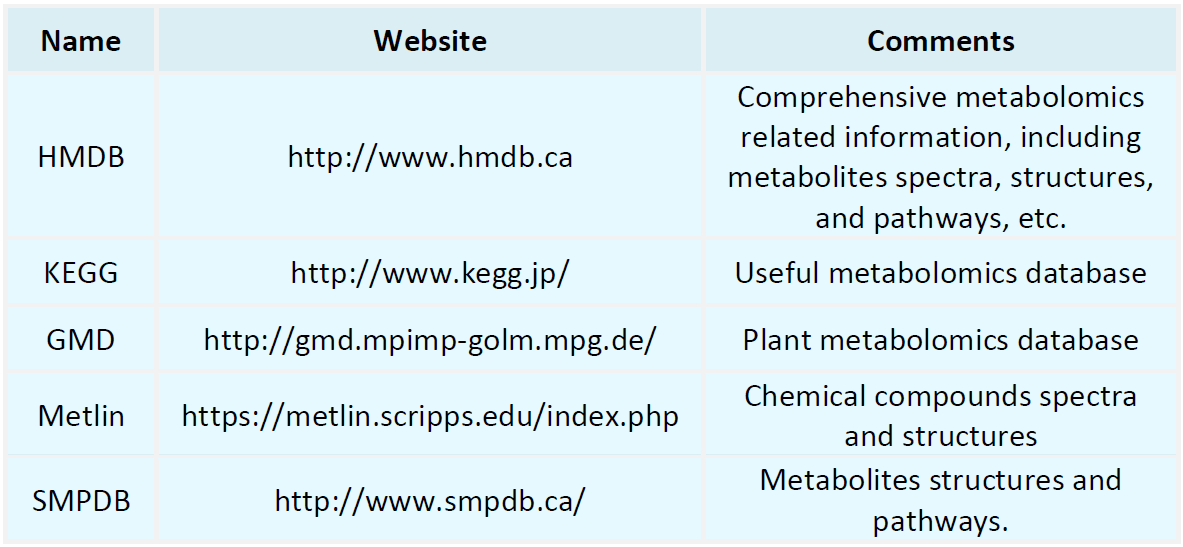
Metabolomics Databases
-
• Isobaric Tag for Relative and Absolute Quantitation
Isobaric Tag for Relative and Absolute Quantitation is an advanced mass spectrometry technique used in proteomics research, enabling the relative or absolute quantification of proteins in various samples through isotope labeling. Isobaric tag for relative and absolute quantitation allows researchers to measure protein expression changes under different experimental conditions, facilitating the exploration of biological processes such as cell signaling, disease mechanisms, and drug responses. Additiona......
-
• GST Pull-Down Assay Principle
The GST pull-down assay is an in vitro experimental method that employs glutathione S-transferase (GST) as a tag to express and isolate target proteins. This technique harnesses the affinity between GST and glutathione agarose beads to separate the fusion protein from complex mixtures. GST pull-down assays are extensively used not only for protein purification but also for investigating protein-protein interactions, screening interacting molecules, and validating target proteins' functions in drug dev......
-
HPLC-MALDI-TOF-MS is an advanced analytical technique integrating HPLC with MALDI-TOF-MS. HPLC is employed to separate components in a mixture by utilizing various stationary and mobile phases in a liquid environment. Complementing this, MALDI-TOF-MS ionizes sample molecules using matrix-assisted laser excitation and determines their mass-to-charge ratios via a time-of-flight analyzer. This method is widely applicable in biomedicine, drug development, food safety, and environmental monitoring. Within ......
-
• Edman Degradation of Protein
Edman degradation of protein is a chemical analysis technique that sequentially cleaves amino acid residues from the N-terminus of a peptide chain, enabling the determination of the protein’s amino acid sequence. The principle of Edman degradation of protein involves the reaction of phenylisothiocyanate with the N-terminal amino acid of the protein to form a phenylthiocarbamyl derivative, which is then cyclized under acidic conditions, releasing PTH-amino acids. Each cycle identifies a single amino ac......
-
• GST Fusion Protein Pull-Down Assay
GST fusion protein pull-down assay is a widely used biochemical technique for studying protein-protein interactions. GST (glutathione S-transferase) is an enzyme that specifically binds to glutathione (GSH). By fusing the target protein with GST, the interaction between GST and GSH can be utilized to purify and separate the protein. The basic principle of the GST fusion protein pull-down assay involves fusing GST with the target protein, expressing it, and immobilizing the fusion protein on GSH resin ......
-
• Cross-Linking Protein Interaction Analysis
Cross-linking protein interaction analysis is a technique used to investigate the physical interactions between proteins and their roles in biological systems. Protein interactions are integral to virtually all biological processes, such as signal transduction, metabolic pathways, and gene expression regulation. By decoding these interactions, scientists can unveil complex cellular networks, which are pivotal for understanding disease mechanisms, developing new drugs, and identifying biomarkers. For i......
-
• Edman Degradation N-Terminal Sequencing
Edman degradation N-terminal sequencing is a well-established method for determining the N-terminal amino acid sequence of protein chains. Developed by Pehr Edman in the 1950s, this technique quickly became a cornerstone in protein chemistry. Through a series of chemical reactions, Edman degradation sequentially removes and identifies N-terminal amino acids, allowing researchers to elucidate the primary structure of proteins over time. In proteomics, understanding a protein’s amino acid sequence is cr......
-
• Co-Immunoprecipitation Pull-Down Assay
Co-immunoprecipitation pull-down assay is an established technique for analyzing protein-protein interactions. This method captures protein complexes interacting with a target protein through specific antibodies binding to it. Widely employed in molecular biology, the assay enables the verification of protein complex existence and composition. For instance, examining interactions between transcription factors and DNA-binding proteins enhances our understanding of gene expression regulation mechanisms.......
-
Collagen analysis by HPLC is a widely utilized technique in proteomics research. The principle of collagen analysis by HPLC lies in the high resolution and sensitivity offered by liquid chromatography. This method involves preparing the sample through dissolution, filtration, and injection into the HPLC system. The sample is then separated within the chromatographic column, where it interacts with the stationary and mobile phases. Each component is progressively eluted based on its partitioning behavi......
-
• Co-Immunoprecipitation and Pull-Down Assays
Co-immunoprecipitation and pull-down assays are pivotal techniques for investigating protein-protein interactions. These methods are fundamental in modern molecular biology and proteomics research, facilitating the exploration of intricate cellular protein networks. Co-IP is predominantly used to verify established protein interactions or to detect unknown interacting partners within the same biological pathway. Pull down assays employ a tagged "bait" protein to isolate "prey" proteins that bind to it......
How to order?







