Resources
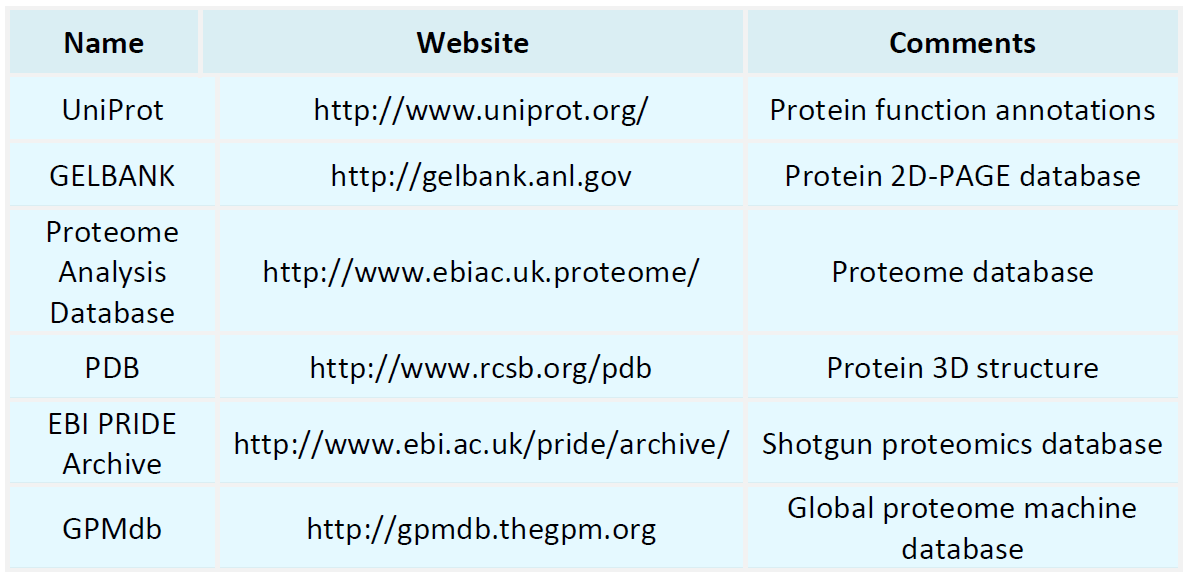
Proteomics Databases
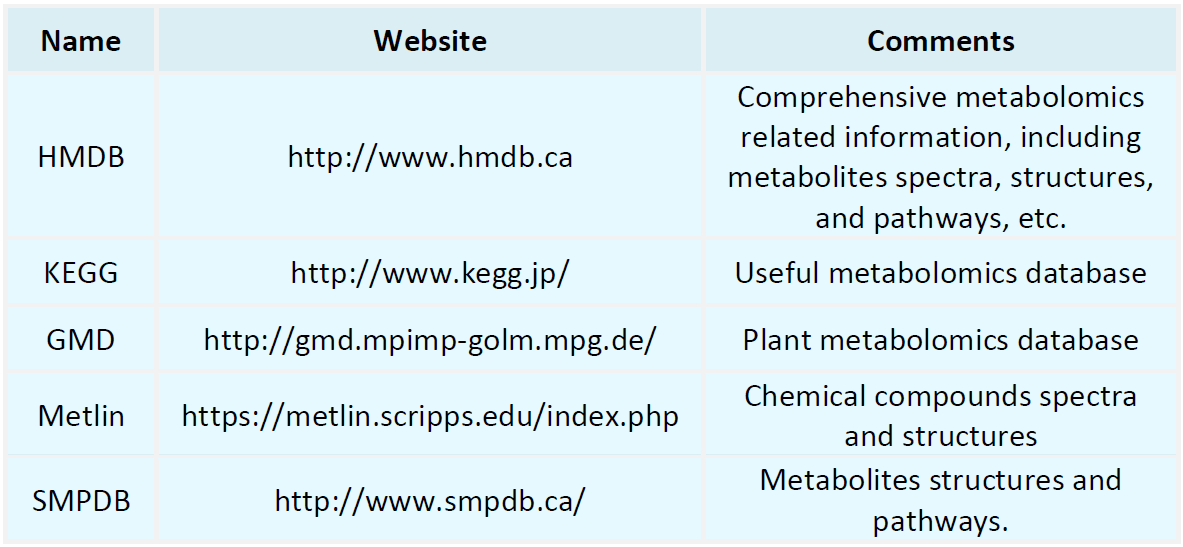
Metabolomics Databases
-
• Optimization of De Novo Protein Sequencing with Mass Spectrometry
De novo protein sequencing is the process of determining the amino acid sequence of a protein without relying on genomic or protein databases, using high-resolution mass spectrometry. Unlike homology-based sequence analysis, which depends on database comparisons, de novo sequencing is particularly valuable for studying proteins from unknown species, characterizing epigenetically modified proteins, and developing antibody-based therapeutics. By analyzing fragment ion spectra of enzymatically digested p......
-
• N-Terminal Sequencing: 8 Common Mistakes You Must Avoid
N-terminal sequencing is widely used to determine the N-terminal amino acid sequence of proteins and peptides. The accuracy of its results is crucial for protein identification, functional annotation, and understanding disease mechanisms. However, even minor deviations in experimental design and execution can lead to distorted data or incorrect conclusions. This article outlines 8 common mistakes in N-terminal sequencing and offers strategies to mitigate them, helping researchers enhance both experime......
-
• Applications of De Novo Protein Sequencing in Proteomics
De novo protein sequencing circumvents these constraints by directly determining the primary amino acid sequence of proteins, establishing itself as a transformative tool in proteomics. Proteomics seeks to systematically characterize the composition, modifications, and functions of proteins in biological systems. However, traditional approaches depend on pre-existing genomic databases, limiting their ability to analyze unknown proteins, complex modifications, and non-model organisms. This review highl......
-
• Experimental Steps and Data Analysis Strategies in De Novo Protein Sequencing
De novo protein sequencing is a mass spectrometry-based technique that enables the direct determination of full-length protein sequences without reference to genomic or protein sequence databases. This approach is widely utilized in the identification of novel biomarkers, antibody drug development, and the characterization of proteins from non-model organisms. It is particularly valuable for analyzing unknown proteins, such as newly discovered biomarkers and antibody variable regions, as well as prote......
-
• Advantages and Limitations of De Novo Protein Sequencing
De novo protein sequencing is an advanced technique for determining protein sequences without requiring genomic data or database references. This approach is particularly valuable for studying unknown proteins, novel antibodies, non-model organisms, and complex post-translational modifications (PTMs). Unlike traditional mass spectrometry-based identification, which relies on database searches, de novo sequencing is especially useful for novel protein discovery and modification analysis. However, chall......
-
• How Does Native MS Revolutionize Structural Biology?
Native MS is an advanced analytical technique that enables the characterization of protein mass, assembly states, and molecular interactions under near-physiological conditions. As structural biology expands from single-protein studies to complex, dynamic multiprotein systems, native MS has emerged as a transformative and complementary approach that enhances conventional structural biology methods. This review systematically examines the critical role of native MS in structural biology, highlights its......
-
• Flow Injection Analysis Mass Spectrometry
Flow injection analysis mass spectrometry (FIA-MS) combines the strengths of flow injection analysis and mass spectrometry, enabling rapid and precise qualitative and quantitative analysis of chemical components in complex samples. The underlying principle of FIA-MS is relatively straightforward. In this method, a liquid sample is introduced into the injection valve through a flow injection system, where it mixes with a carrier solvent before entering the mass spectrometer under controlled conditions.......
-
• Receptor Identification and Characterization
Receptor identification and characterization techniques are extensively utilized in modern biological research fields, including drug development, disease studies, and fundamental biological investigations. Receptors, which are proteins located on the cell surface or within cells, are responsible for detecting and binding specific signaling molecules-such as hormones, neurotransmitters, and drugs-thereby initiating biological effects. Within biological systems, receptors function akin to switches, con......
-
• Protein Primary Structure Analysis
Protein primary structure analysis is a fundamental step in protein research, focusing on determining and understanding the amino acid sequence of proteins. As essential functional molecules within organisms, proteins perform a wide range of biological activities. Their primary structure, the specific order of amino acids, is crucial for elucidating their function and properties. Analyzing this structure provides insight into the protein's conformation, function, and interactions with other molecules.......
-
Thermal shift analysis (TSA) is a technique employed to evaluate protein stability and ligand-binding characteristics by assessing protein conformational changes at varying temperatures. Typically, proteins denature upon heating, which induces measurable structural changes. Thermal shift analysis (TSA) leverages the interaction between fluorescent dyes and proteins; the fluorescence intensity of these dyes changes upon binding to denatured proteins. By examining the temperature-dependent fluorescence ......
How to order?







