Resources
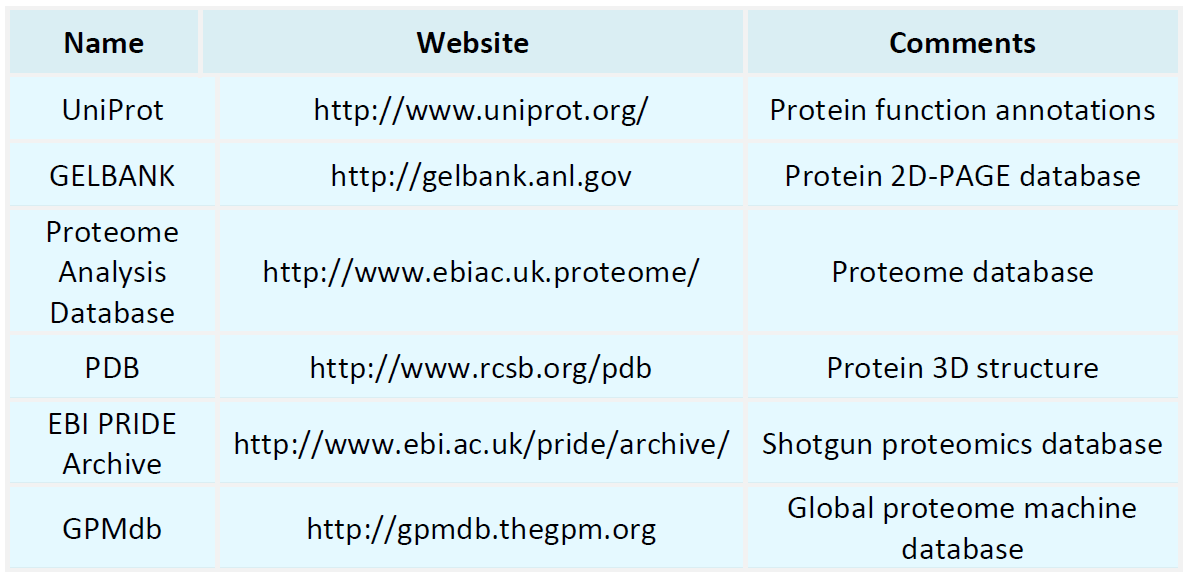
Proteomics Databases
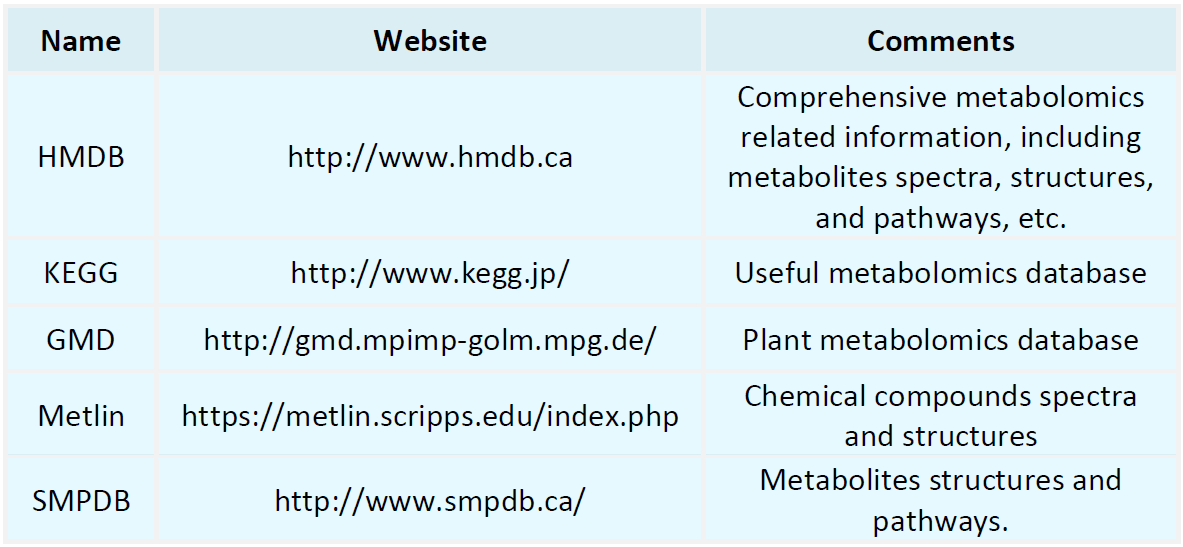
Metabolomics Databases
-
• From Failure to Precision: Common Challenges and Optimization Strategies in Peptide Sequencing
Peptide sequencing, utilizing high-resolution mass spectrometry combined with bioinformatic analysis, allows researchers to elucidate amino acid sequences, characterize post-translational modifications (PTMs), and monitor dynamic proteomic changes. As a pivotal technique in proteomics, it has extensive applications in protein identification, PTM profiling, neoantigen discovery, and drug development. However, suboptimal or failed results are frequently encountered due to variables such as sample integr......
-
• Advantages and Disadvantages of Shotgun Proteomics
Shotgun proteomics represents a widely adopted strategy for comprehensive proteome identification in life science research. By enzymatically digesting all proteins within a sample into peptides and leveraging liquid chromatography coupled with high-resolution mass spectrometry, this approach enables systematic detection and quantification of proteins in complex biological matrices. This review aims to systematically evaluate the principal strengths and inherent limitations of shotgun proteomics to ass......
-
• Comprehensive Disulfide Bond Analysis in Antibody Therapeutics
Immunoglobulins (Ig), commonly referred to as antibodies, are a class of highly complex glycoproteins composed of two heavy chains and two light chains. Their structural stability is governed not only by non-covalent interactions but more critically by covalent disulfide bonds. These disulfide linkages can be classified as intrachain or interchain and are essential for preserving the antibody’s native three-dimensional conformation, structural integrity, and biological activity. In therapeutic monoclo......
-
• The Pivotal Role of Glycosylation Profiling in Drug Development
Glycosylation, one of the most prevalent and complex post-translational modifications of proteins, plays a vital role in determining the structure and function of biological macromolecules. In recent years, the large-scale development of biotherapeutics, such as monoclonal antibodies and recombinant protein drugs, has highlighted the strategic importance of glycosylation profiling throughout the drug development process. Glycosylation Influences Drug Efficacy, Safety, and Stability Even subtle variat......
-
• How to Perform High-Throughput Shotgun Protein Identification via LC-MS/MS?
To realize high-throughput shotgun protein identification, liquid chromatography–tandem mass spectrometry (LC–MS/MS) has emerged as one of the most widely adopted and efficient analytical strategies. This technology enables the rapid identification and quantification of thousands of proteins in complex biological samples and is extensively applied in fundamental research, disease mechanism elucidation, and drug target discovery. What Is Shotgun Proteomics? Shotgun proteomics, also referred to as bott......
-
• The Impact of LC-MS-Based Quantitative Analysis on Antibody Characterization
Antibodies are among the most pivotal molecules in contemporary biopharmaceutical research and development. Given their structural complexity and functional diversity, precise characterization is essential in drug development, mechanistic studies, and quality control. Traditionally, antibody analysis has relied on methods such as enzyme-linked immunosorbent assay (ELISA), capillary electrophoresis (CE), and Western blotting. While technically straightforward, these methods present clear limitations in......
-
• What Is Shotgun Protein Identification and How Does It Differ from Traditional Methods?
Shotgun protein identification is a large-scale protein identification strategy that involves enzymatically digesting complex protein mixtures (commonly using trypsin) into peptides, followed by liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis. The resulting spectra are then searched against protein databases to infer the identities of the original proteins. The term shotgun refers to its non-targeted analytical approach; rather than analyzing proteins one by one, it comprehensively ......
-
• Strategic Considerations in the Design of ABPP Probes
Activity-Based Protein Profiling (ABPP) is a functional proteomics approach designed to selectively capture enzymatically active proteins within cells or tissues. Beyond offering protein expression profiles, ABPP provides insight into the actual functional state of enzymes, making it a critical tool for target identification, elucidation of drug mechanisms, and biomarker discovery. Central to the success of ABPP is the rational design of chemical probes. An effective ABPP probe must exhibit high targe......
-
• How to Perform High-Throughput Protein Acylation Analysis?
Protein acylation represents a major class of post-translational modifications (PTMs) that play crucial roles in regulating cellular metabolism, signal transduction, and chromatin remodeling. Recent advances in proteomics, particularly in high-resolution mass spectrometry, have enabled high-throughput profiling of protein acylation. This capability has driven rapid progress in research areas such as metabolic regulation, epigenetics, and biomarker discovery in cancer. What Is Protein Acylation? Prote......
-
Peptides serve as critical intermediaries linking protein functions to various biological processes. They play increasingly significant roles in biomarker discovery, immunotherapy target identification, and natural product research. Peptide sequencing refers to analytical methods used to determine the amino acid sequence of peptide molecules. Composed of 20 standard amino acids linked via peptide bonds, peptides exhibit diverse biological functions, stability profiles, and binding affinities depending......
How to order?







