Resources
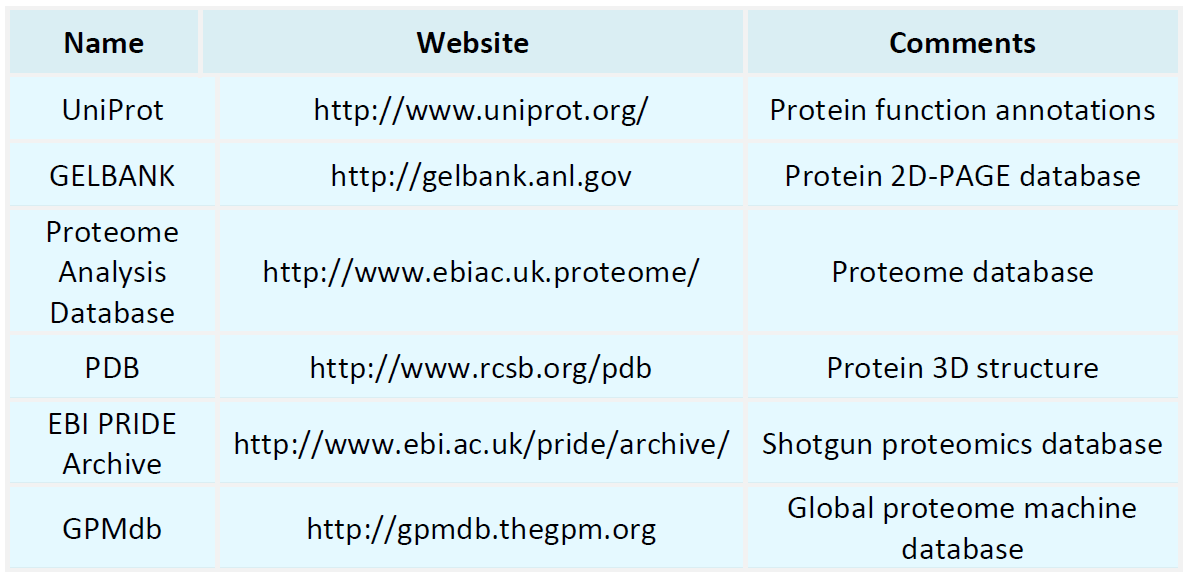
Proteomics Databases
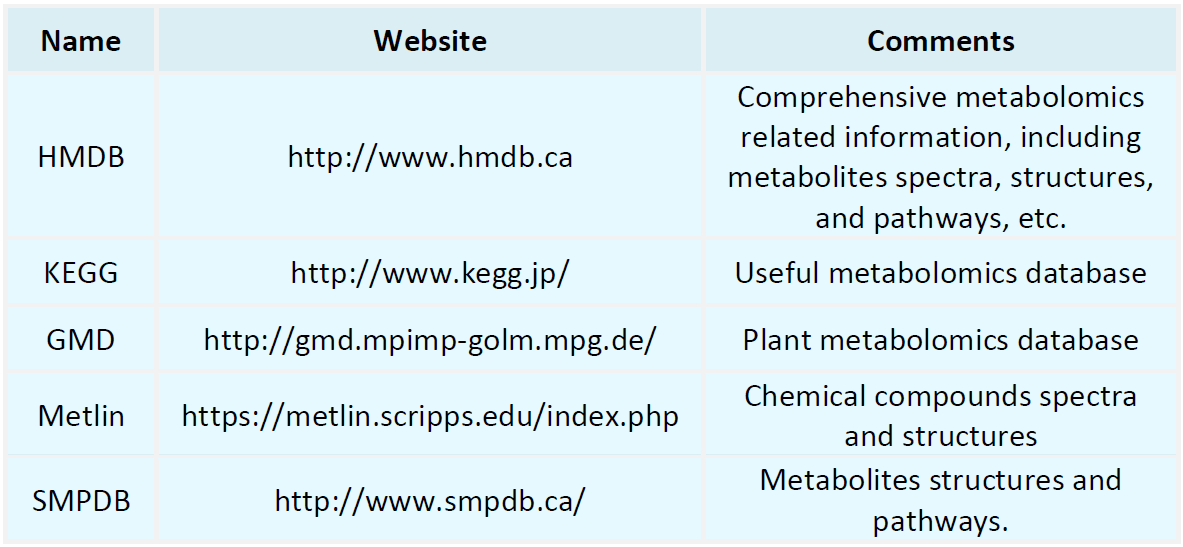
Metabolomics Databases
-
The protein structure is crucial for their function and activity. Circular dichroism spectroscopy is widely used in the field of protein structure analysis and serves as a powerful tool for quality control of biopharmaceuticals.
-
Protein ms identification diagram is an advanced technique that provides us with a window to deeply understand the composition and interactions of proteins. Understanding and identifying the structure and function of proteins is of great significance for disease treatment.
-
• Methods for Determining Primary Structure of Proteins
The primary structure of a protein, namely the amino acid sequence, carries the protein's function and characteristics. Determination primary structure of proteins is of great significance for understanding life processes and developing bioproducts.
-
• Decoding the Mysteries of Proteins in Organisms through Proteomics Sequencing
Proteomics sequencing technology is a series of methods that efficiently and rapidly determine the composition and characteristics of proteins. It plays an important role in deciphering the mysteries of protein in living organisms.
-
• Analyzing Protein Function and Structure via Protein Primary Structure Determination
Protein structure can be divided into four levels: primary structure, secondary structure, tertiary structure, and quaternary structure. Among these levels, protein primary structure is the most fundamental, referring to the amino acid sequence of the protein.
-
• Proteome Analysis Based on De Novo Sequencing
In protein analysis, de novo sequencing, as a powerful analytical method, provides us a new perspective and new opportunities for discoveries.Proteins are the fundamental functional units in living organisms and have critical significance in studying the structure and function of biological drugs.
-
• The Effects of Different Proteomics Sequencing Methods on Research Outcomes
Proteomics sequencing, as a core technique in proteomics research, provides abundant information to reveal the complete profile of proteins in biological systems. However, the choice of different methods in proteomic sequencing can significantly impact research results.
-
• Protein Secondary Structure Analysis and Circular Dichroism
Circular dichroism spectroscopy is commonly used to analyze and interpret the secondary structure of proteins. Through circular dichroism, we can obtain valuable information on protein secondary structure and gain a deeper understanding of the relationship between the folding state and function of proteins.
-
• How to Select and Optimize Protein Sequencing Methods?
Protein sequencing is an important analytical technique in the field of bioproducts, which can reveal the amino acid sequence and structural information of proteins, providing key data for protein research and biopharmaceutical development. However, when choosing and optimizing protein sequencing methods, scientists need to consider multiple factors.
-
• Analysis of Protein Structure Using Circular Dichroism HT Technology
Analysis of protein structure is a critical component in the development and production of biologics, and it holds significant importance in understanding protein function and properties. In the field of protein structure analysis for biologic products, high-throughput (HT) circular dichroism spectroscopy (CD) technology is widely utilized.
How to order?







