Resources
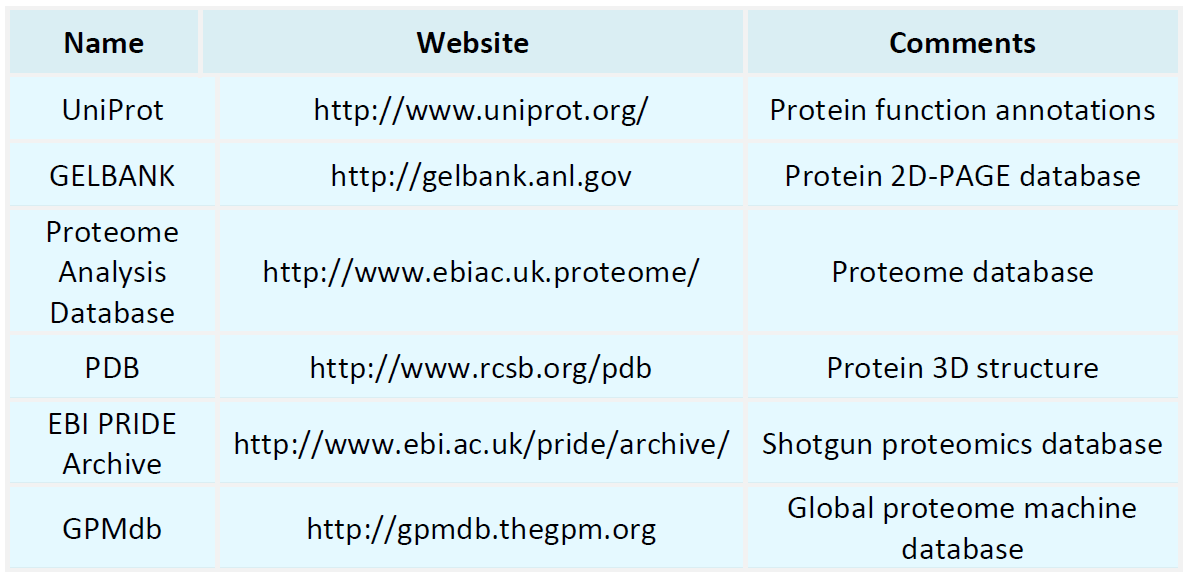
Proteomics Databases
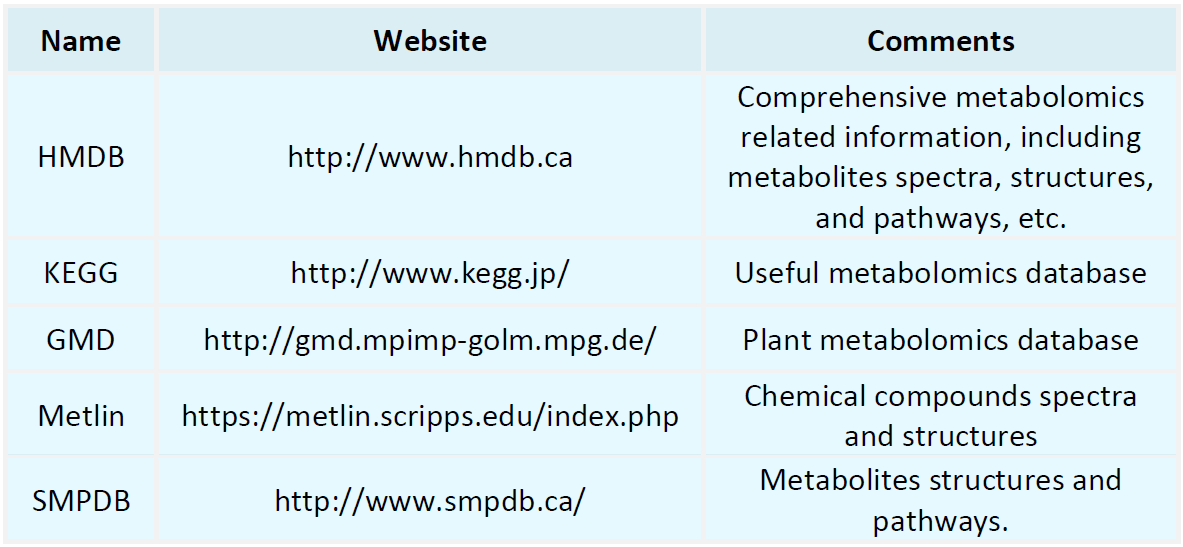
Metabolomics Databases
-
• Revealing the Biological Functions of Carbohydrates Through Polysaccharideomics
Polysaccharideomics is an important research field focused on the study of polysaccharides, which play a crucial role in biological systems. Traditionally, researchers have primarily focused on proteins and nucleic acids, paying less attention to carbohydrates. However, with the rise of polysaccharideomics, we have gradually realized the significance of carbohydrates in cell signaling, disease occurrence, and drug development.
-
• The Role of Circular Dichroism in Determining Protein Secondary Structure
Understanding the secondary structure of proteins is crucial for unraveling their functions and properties. In the field of protein structure in biopharmaceuticals, circular dichroism spectroscopy is widely used to determine protein secondary structure and plays a key role in unraveling the folding mysteries of proteins.
-
• Protein Mass Spectrometry Quantification: Revealing a Complete Picture of Protein Expression
Proteins are among the most important molecules in biological systems for their diversity and functionality. To understand the regulatory mechanisms and functional features of biological systems, it is critical to reveal the full picture of protein expression. Protein mass spectrometry quantification can quantitatively analyze abundance changes in large-scale protein samples, thereby providing comprehensive information about protein expression. Importance of Protein MS Quantification 1. Understandin......
-
• Differential Protein Analysis Promoting New Drug Development
Differential protein analysis has become a powerful tool for better understanding the molecular mechanisms of disease development and discovering effective therapeutic targets.
-
• Applications and Innovations in Oligosaccharide Analysis Techniques
Oligosaccharides, as an important class of bioactive molecules, play a crucial role in the field of biopharmaceuticals. With advancements in technology and scientific development, breakthroughs have been made in oligosaccharide analysis techniques, opening up new avenues for a deeper understanding of their characteristics and applications.
-
• Does the Low-Purity Protein Identification Affect the Accuracy of Research Results?
Protein identification is an important aspect in biological and biomedical research, as it helps scientists understand the structure, function, and disease-related mechanisms of proteins. However, low purity of proteins during identification can impact the accuracy of results.
-
• Commonly Used Methods for Protein Differential Analysis
Proteins are an essential part of living organisms, playing a pivotal role in the regulation and function of life processes. In biomedical research and drug development, protein differential analysis is crucial for revealing disease mechanisms and discovering new treatment targets.
-
• Decrypting Protein Spatial Conformation through Protein Secondary Structure Analysis
Protein is one of the most functionally diverse molecules in living organisms, and its function is closely related to its spatial conformation. The secondary structure of proteins refers to the repetitive folding patterns in local regions of the polypeptide chain, including α-helix, β-sheet, and random coil. By analyzing the secondary structure of proteins, we can uncover their function and interactions, providing an important theoretical basis for fields such as biology and drug development. Defini......
-
• Proteomic Study of Protein Phosphorylation Using Mass Spectrometry Technology
Protein phosphorylation is a common and important epigenetic modification that regulates cellular signaling and function. With technological advancements, mass spectrometry has become an important tool for proteomic study of protein phosphorylation. Proteomic study of protein phosphorylation using mass spectrometry allows for comprehensive and high-throughput identification and quantification of phosphorylation sites in cells, revealing the regulatory mechanisms of phosphorylation networks and biological pr
-
• Significance and Methods of Protein Sequence Characterization
Protein sequence characterization is a crucial method for analyzing and interpreting protein sequences, which contain information about their structure and function, making it a significant molecule in organisms.
How to order?







