Resources
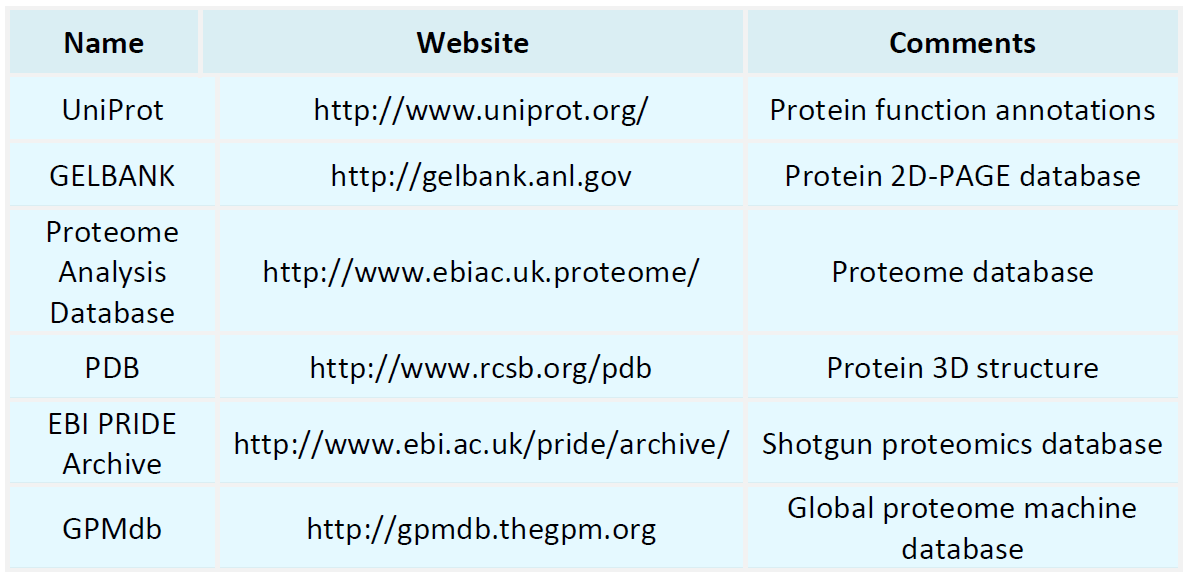
Proteomics Databases
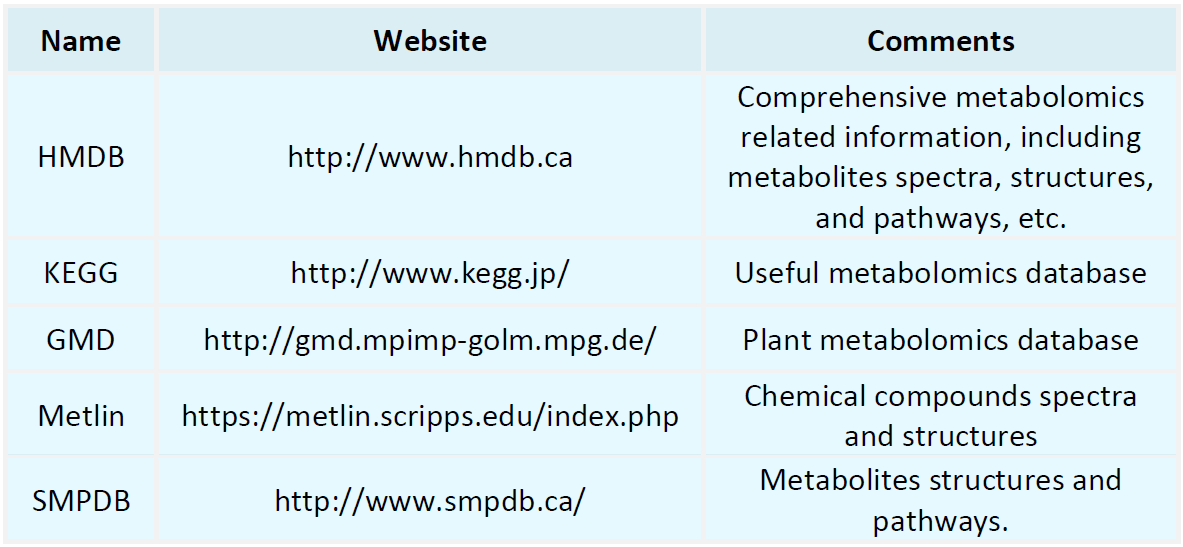
Metabolomics Databases
-
Oligosaccharide profiling is aimed at thoroughly analyzing the complex structures of oligosaccharides to elucidate their biological functions and roles. Oligosaccharides, composed of a limited number of monosaccharides linked by glycosidic bonds, are prevalent on the surfaces of animal and plant cells and are involved in various biological processes such as cell recognition, immune response, and signal transduction. By comprehensively examining their structures, compositions, and modifications, oligos......
-
• Protein Sequencing Technology
Protein sequencing technology is fundamental to determining the amino acid sequences of proteins, providing critical insights into their structure-function relationships. This technology is widely applied in medicine, drug discovery, and biotechnology. In disease research, protein sequencing aids in identifying disease-associated protein variants, supporting early diagnosis and personalized treatment. Furthermore, sequencing specific proteins enables scientists to design engineered proteins with tailo......
-
• C-Terminal Protein Sequencing in Biopharmaceuticals
C-Terminal protein sequencing analysis is a technique that precisely analyzes the carboxyl-terminal sequence of proteins or polypeptides. This technique is crucial for identifying C-terminal amino acid sequences, detecting modifications at the C-terminal, and confirming the completeness of protein processing. The C-terminal sequences play significant roles in various biological processes, including the regulation of protein stability, functional activity, interactions, and signal transduction. Moreove......
-
Site-specific analysis is employed to investigate genetic or protein sequence variations at the molecular level at specific locations. Its core principle is to identify and analyze variations at precise positions in specific genes or proteins, facilitating insight into gene function, disease mechanisms, and the development of personalized treatment strategies. This method concentrates on the detailed examination of characteristics and changes at specific sites within biological macromolecules (such as......
-
• SDS-PAGE Protein Quantification
SDS-PAGE protein quantification analysis is extensively utilized in biochemical and molecular biology research. SDS-PAGE, which stands for Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis, is a technique that separates proteins in a sample for analytical purposes. SDS, an anionic detergent, binds to protein molecules, disrupts their native conformation, and confers a uniform negative charge, thereby enabling their migration in an electric field. This technique is broadly employed for protein ......
-
Biosimilar characterization involves a thorough and systematic analysis of the physicochemical properties, structural features, functional activity, and safety profiles of biosimilars. This ensures they are highly similar to their reference biologics in terms of quality, safety, and efficacy. Biosimilars, which include complex proteins like monoclonal antibodies, fusion proteins, and recombinant proteins, require meticulous production involving cell cultures and protein purification. Even minor change......
-
• Lipid Analysis by Mass Spectrometry
Lipid analysis by mass spectrometry is an indispensable technique for elucidating the composition, structure, and function of lipids. Lipids-including fats, phospholipids, and sphingolipids-are vital for cellular architecture, energy storage, and signal transduction. In lipid analysis by mass spectrometry, lipid molecules are ionized and their mass-to-charge ratios (m/z) are measured, enabling precise determination of molecular masses and structural features. This method is critical for unraveling com......
-
• Expression Analysis and Characterization of Protein
The expression analysis and characterization of protein encompass the identification, quantification, and functional investigation of proteins within cells or tissues. Proteins, as the primary executors of biological functions, have expression levels and functional statuses that crucially determine the physiological state and responsiveness of cells. This process involves several key aspects. Firstly, quantitative analysis of proteins allows researchers to identify proteins associated with specific fu......
-
• Functional Characterization of Proteins
Functional characterization of proteins seeks to uncover the functions and mechanisms of specific proteins within organisms. Proteins are fundamental to life, participating in nearly all biological processes, such as metabolism, signal transduction, immune responses, and cellular structure maintenance. In the realm of proteomics, understanding protein functions aids scientists in unraveling the complexities of biological systems. This methodology also elucidates protein interactions, regulatory pathwa......
-
• N-Terminal Sequencing Method
The N-Terminal sequencing method is primarily employed to ascertain the amino acid sequence at the N-terminus of proteins or peptides. This method encompasses techniques such as Edman degradation and mass spectrometry analysis. First introduced in the 1950s, the Edman degradation technique determines sequences by systematically removing and identifying the N-terminal amino acids of a peptide chain. Noted for its high specificity and accuracy, it is particularly effective for analyzing short-chain pept......
How to order?







